Antifibrotics Modify B-Cell-induced Fibroblast Migration and Activation in Patients with Idiopathic Pulmonary Fibrosis
- PMID: 33689587
- PMCID: PMC8456878
- DOI: 10.1165/rcmb.2020-0387OC
Antifibrotics Modify B-Cell-induced Fibroblast Migration and Activation in Patients with Idiopathic Pulmonary Fibrosis
Abstract
B-cell activation is increasingly linked to numerous fibrotic lung diseases, and it is well known that aggregates of lymphocytes form in the lung of many of these patients. Activation of B-cells by pattern recognition receptors (PRRs) drives the release of inflammatory cytokines, chemokines, and metalloproteases important in the pathophysiology of pulmonary fibrosis. However, the specific mechanisms of B-cell activation in patients with idiopathic pulmonary fibrosis (IPF) are poorly understood. Herein, we have demonstrated that B-cell activation by microbial antigens contributes to the inflammatory and profibrotic milieu seen in patients with IPF. B-cell stimulation by CpG and β-glucan via PRRs resulted in activation of mTOR-dependent and independent pathways. Moreover, we showed that the B-cell-secreted inflammatory milieu is specific to the inducing antigen and causes differential fibroblast migration and activation. B-cell responses to infectious agents and subsequent B-cell-mediated fibroblast activation are modifiable by antifibrotics, but each seems to exert a specific and different effect. These results suggest that, upon PRR activation by microbial antigens, B-cells can contribute to the inflammatory and fibrotic changes seen in patients with IPF, and antifibrotics are able to at least partially reverse these responses.
Keywords: B-lymphocytes; antifibrotics; fibrosis; idiopathic pulmonary fibrosis; pattern recognition receptor.
Figures

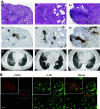


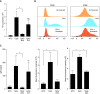
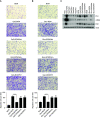
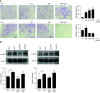
Comment in
-
B Cells in Idiopathic Pulmonary Fibrosis: Targeting Immune Cells with Antifibrotic Agents.Am J Respir Cell Mol Biol. 2021 Jun;64(6):652-654. doi: 10.1165/rcmb.2021-0101ED. Am J Respir Cell Mol Biol. 2021. PMID: 33725473 Free PMC article. No abstract available.
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. - PubMed
-
- King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

