Drp1 is required for AgRP neuronal activity and feeding
- PMID: 33689681
- PMCID: PMC7946429
- DOI: 10.7554/eLife.64351
Drp1 is required for AgRP neuronal activity and feeding
Erratum in
-
Correction: Drp1 is required for AgRP neuronal activity and feeding.Elife. 2022 Jun 28;11:e80570. doi: 10.7554/eLife.80570. Elife. 2022. PMID: 35763333 Free PMC article.
Abstract
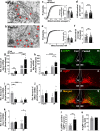
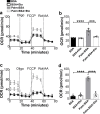
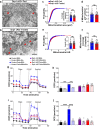
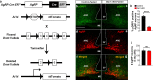
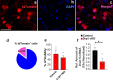
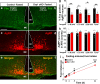
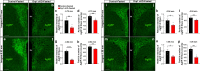
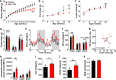
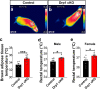
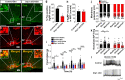
The hypothalamic orexigenic Agouti-related peptide (AgRP)-expressing neurons are crucial for the regulation of whole-body energy homeostasis. Here, we show that fasting-induced AgRP neuronal activation is associated with dynamin-related peptide 1 (DRP1)-mediated mitochondrial fission and mitochondrial fatty acid utilization in AgRP neurons. In line with this, mice lacking Dnm1l in adult AgRP neurons (Drp1 cKO) show decreased fasting- or ghrelin-induced AgRP neuronal activity and feeding and exhibited a significant decrease in body weight, fat mass, and feeding accompanied by a significant increase in energy expenditure. In support of the role for mitochondrial fission and fatty acids oxidation, Drp1 cKO mice showed attenuated palmitic acid-induced mitochondrial respiration. Altogether, our data revealed that mitochondrial dynamics and fatty acids oxidation in hypothalamic AgRP neurons is a critical mechanism for AgRP neuronal function and body-weight regulation.
Keywords: AgRP; feeding; metabolism; mitochondria; mouse; neuroscience.
© 2021, Jin et al.
Conflict of interest statement
SJ, NY, ZL, JS, TH, JK, SD No competing interests declared
Figures






References
-
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

