COVID-19 test result reporting for deceased donors: Emergent policies, logistical challenges, and future directions
- PMID: 33690910
- PMCID: PMC8206716
- DOI: 10.1111/ctr.14280
COVID-19 test result reporting for deceased donors: Emergent policies, logistical challenges, and future directions
Abstract
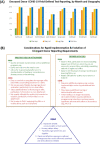
The coronavirus disease 2019 (COVID-19) pandemic poses unprecedented challenges to the transplant community, including organ procurement organizations (OPOs), transplant centers, regulatory agencies, and recipient candidates. Access to timely, accurate information on the status of deceased donor viral infection is essential in determining organ acceptance. The Organ Procurement and Transplantation Network expeditiously added fields to collect these data; however, use of the data collection fields was not uniform nationally. Standardized, field-defined data capture and reporting are vital to ensure optimal organ utilization during this pandemic, and to prepare the community for subsequent challenges.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no relevant conflicts of interest or other relevant financial disclosures. All authors approve and agree to be accountable for ensuring the accuracy and integrity of the final manuscript.
Figures
References
-
- Organ Procurement and Transplantation Network (OPTN) . COVID‐19 Emergency Policies and Data Collection. Available at: https://optn.transplant.hrsa.gov/governance/public‐comment/ Accessed: 1/11/2021
-
- Organ Procurement and Transplantation Network (OPTN) . COVID‐19 Policy Actions Implemented. Available at: https://optn.transplant.hrsa.gov/governance/policy‐notices. Accessed: 1/11/2021
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

