Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine
- PMID: 33691060
- PMCID: PMC8008743
- DOI: 10.1056/NEJMc2101667
Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine
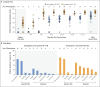
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
