CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability
- PMID: 33691090
- PMCID: PMC8102368
- DOI: 10.1016/j.cmet.2021.02.015
CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability
Abstract
Understanding the mechanisms underlying how T cells become dysfunctional in a tumor microenvironment (TME) will greatly benefit cancer immunotherapy. We found that increased CD36 expression in tumor-infiltrating CD8+ T cells, which was induced by TME cholesterol, was associated with tumor progression and poor survival in human and murine cancers. Genetic ablation of Cd36 in effector CD8+ T cells exhibited increased cytotoxic cytokine production and enhanced tumor eradication. CD36 mediated uptake of fatty acids by tumor-infiltrating CD8+ T cells in TME, induced lipid peroxidation and ferroptosis, and led to reduced cytotoxic cytokine production and impaired antitumor ability. Blocking CD36 or inhibiting ferroptosis in CD8+ T cells effectively restored their antitumor activity and, more importantly, possessed greater antitumor efficacy in combination with anti-PD-1 antibodies. This study reveals a new mechanism of CD36 regulating the function of CD8+ effector T cells and therapeutic potential of targeting CD36 or inhibiting ferroptosis to restore T cell function.
Keywords: CD36; CD8(+) T cells; ferroptosis; lipid peroxidation.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

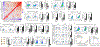
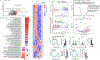
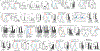
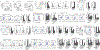

Comment in
-
CD36 pumps fat to defang killer T cells in tumors.Cell Metab. 2021 Aug 3;33(8):1509-1511. doi: 10.1016/j.cmet.2021.07.004. Cell Metab. 2021. PMID: 34348095
References
-
- ALMEIDA L, LOCHNER M, BEROD L. & SPARWASSER T. 2016. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol, 28, 514–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

