The methodological quality is insufficient in clinical practice guidelines in the context of COVID-19: systematic review
- PMID: 33691153
- PMCID: PMC7937325
- DOI: 10.1016/j.jclinepi.2021.03.005
The methodological quality is insufficient in clinical practice guidelines in the context of COVID-19: systematic review
Abstract
Objectives: The number of published clinical practice guidelines related to COVID-19 has rapidly increased. This study explored if basic methodological standards of guideline development have been met in the published clinical practice guidelines related to COVID-19.
Study design and setting: Rapid systematic review from February 1 until April 27, 2020 using MEDLINE [PubMed], CINAHL [Ebsco], Trip and manual search, including all types of healthcare workers providing any kind of healthcare to any patient population in any setting.
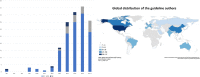
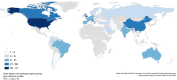
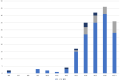
Results: There were 1342 titles screened and 188 guidelines included. The highest average AGREE II domain score was 89% for scope and purpose, the lowest for rigor of development (25%). Only eight guidelines (4%) were based on a systematic literature search and a structured consensus process by representative experts (classified as the highest methodological quality). The majority (156; 83%) was solely built on an informal expert consensus. A process for regular updates was described in 27 guidelines (14%). Patients were included in the development of only one guideline.
Conclusion: Despite clear scope, most publications fell short of basic methodological standards of guideline development. Clinicians should use guidelines that include up-to-date information, were informed by stakeholder involvement, and employed rigorous methodologies.
Keywords: COVID-19; Clinical practice guidelines; Coronavirus; Guidelines; Recommendations; SARS CoV-2.
Copyright © 2021. Published by Elsevier Inc.
Figures

References
-
- World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected—interim guidance. World Health Organization. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-ac.... 2020. Accessed April 5, 2020.
-
- American Academy of Family Physicians (AAFP). Clinical practice guideline manual. Available at: https://www.aafp.org/patient-care/clinical-recommendations/cpg-manual.html. 2017. Accessed May 22, 2020.
-
- Institute of Medicine . In: Clinical practice guidelines we can trust. Graham R, Mancher M., editors. National Academies Press; Washington, DC: 2011. Committee on standards for developing trustworthy clinical practice guidelines. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

