Cell-type-specific imaging of neurotransmission reveals a disrupted excitatory-inhibitory cortical network in isoflurane anaesthesia
- PMID: 33691246
- PMCID: PMC7941179
- DOI: 10.1016/j.ebiom.2021.103272
Cell-type-specific imaging of neurotransmission reveals a disrupted excitatory-inhibitory cortical network in isoflurane anaesthesia
Abstract
Background: Despite the fundamental clinical significance of general anaesthesia, the cortical mechanism underlying anaesthetic-induced loss of consciousness (aLOC) remains elusive.
Methods: Here, we measured the dynamics of two major cortical neurotransmitters, gamma-aminobutyric acid (GABA) and glutamate, through in vivo two-photon imaging and genetically encoded neurotransmitter sensors in a cell type-specific manner in the primary visual (V1) cortex.
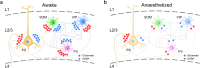
Findings: We found a general decrease in cortical GABA transmission during aLOC. However, the glutamate transmission varies among different cortical cell types, where in it is almost preserved on pyramidal cells and is significantly reduced on inhibitory interneurons. Cortical interneurons expressing vasoactive intestinal peptide (VIP) and parvalbumin (PV) specialize in disinhibitory and inhibitory effects, respectively. During aLOC, VIP neuronal activity was delayed, and PV neuronal activity was dramatically inhibited and highly synchronized.
Interpretation: These data reveal that aLOC resembles a cortical state with a disrupted excitatory-inhibitory network and suggest that a functional inhibitory network is indispensable in the maintenance of consciousness.
Funding: This work was supported by the grants of the National Natural Science Foundation of China (grant nos. 81620108012 and 82030038 to H.D. and grant nos. 31922029, 61890951, and 61890950 to J.H.).
Keywords: GABA glutamate neurotransmission anaesthesia consciousness cortex.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interests The authors declare no competing interests.
Figures




Comment in
-
GABA inhibitory network: A requirement of maintenance of consciousness.EBioMedicine. 2021 Jun;68:103330. doi: 10.1016/j.ebiom.2021.103330. Epub 2021 Jun 14. EBioMedicine. 2021. PMID: 34139433 Free PMC article. No abstract available.
References
-
- Meyer K. The role of dendritic signaling in the anesthetic suppression of consciousness. Anesthesiology. 2015;122(6):1415–1431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

