Prosaposin mediates inflammation in atherosclerosis
- PMID: 33692130
- PMCID: PMC8209679
- DOI: 10.1126/scitranslmed.abe1433
Prosaposin mediates inflammation in atherosclerosis
Abstract
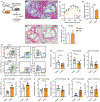
Macrophages play a central role in the pathogenesis of atherosclerosis. The inflammatory properties of these cells are dictated by their metabolism, of which the mechanistic target of rapamycin (mTOR) signaling pathway is a key regulator. Using myeloid cell-specific nanobiologics in apolipoprotein E-deficient (Apoe -/-) mice, we found that targeting the mTOR and ribosomal protein S6 kinase-1 (S6K1) signaling pathways rapidly diminished plaque macrophages' inflammatory activity. By investigating transcriptome modifications, we identified Psap, a gene encoding the lysosomal protein prosaposin, as closely related with mTOR signaling. Subsequent in vitro experiments revealed that Psap inhibition suppressed both glycolysis and oxidative phosphorylation. Transplantation of Psap -/- bone marrow to low-density lipoprotein receptor knockout (Ldlr -/-) mice led to a reduction in atherosclerosis development and plaque inflammation. Last, we confirmed the relationship between PSAP expression and inflammation in human carotid atherosclerotic plaques. Our findings provide mechanistic insights into the development of atherosclerosis and identify prosaposin as a potential therapeutic target.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


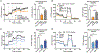

References
-
- Ketelhuth DFJ, Lutgens E, Bäck M, Binder CJ, Van Den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O’Neill L, Weber C, Evans PC, Immunometabolism and atherosclerosis: Perspectives and clinical significance: A position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology, Cardiovasc. Res 115, 1385–1392 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

