Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice
- PMID: 33692134
- PMCID: PMC8830379
- DOI: 10.1126/scitranslmed.aay9056
Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice
Abstract
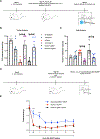
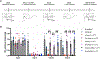
Current treatments for chronic pain rely largely on opioids despite their substantial side effects and risk of addiction. Genetic studies have identified in humans key targets pivotal to nociceptive processing. In particular, a hereditary loss-of-function mutation in NaV1.7, a sodium channel protein associated with signaling in nociceptive sensory afferents, leads to insensitivity to pain without other neurodevelopmental alterations. However, the high sequence and structural similarity between NaV subtypes has frustrated efforts to develop selective inhibitors. Here, we investigated targeted epigenetic repression of NaV1.7 in primary afferents via epigenome engineering approaches based on clustered regularly interspaced short palindromic repeats (CRISPR)-dCas9 and zinc finger proteins at the spinal level as a potential treatment for chronic pain. Toward this end, we first optimized the efficiency of NaV1.7 repression in vitro in Neuro2A cells and then, by the lumbar intrathecal route, delivered both epigenome engineering platforms via adeno-associated viruses (AAVs) to assess their effects in three mouse models of pain: carrageenan-induced inflammatory pain, paclitaxel-induced neuropathic pain, and BzATP-induced pain. Our results show effective repression of NaV1.7 in lumbar dorsal root ganglia, reduced thermal hyperalgesia in the inflammatory state, decreased tactile allodynia in the neuropathic state, and no changes in normal motor function in mice. We anticipate that this long-lasting analgesia via targeted in vivo epigenetic repression of NaV1.7 methodology we dub pain LATER, might have therapeutic potential in management of persistent pain states.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
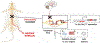



Comment in
-
CRISPR-based gene therapy dampens pain in mice.Nature. 2021 Mar;591(7850):359. doi: 10.1038/d41586-021-00644-5. Nature. 2021. PMID: 33712750 No abstract available.
References
-
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH, The prevalence of chronic pain in United States adults: Results of an internet-based survey. J. Pain 11, 1230–1239 (2010). - PubMed
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D, Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 10, 287–333 (2006). - PubMed
-
- de Leon-Casasola OA, Opioids for chronic pain: New evidence, new strategies, safe prescribing. Am. J. Med. 126, S3–S11 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

