Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2
- PMID: 33692203
- PMCID: PMC8139686
- DOI: 10.1128/JVI.00083-21
Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2
Abstract
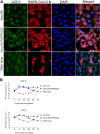
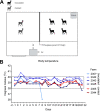
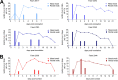
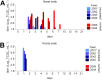
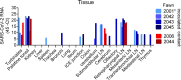
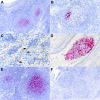
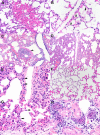
The origin of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing the global coronavirus disease 19 (COVID-19) pandemic, remains a mystery. Current evidence suggests a likely spillover into humans from an animal reservoir. Understanding the host range and identifying animal species that are susceptible to SARS-CoV-2 infection may help to elucidate the origin of the virus and the mechanisms underlying cross-species transmission to humans. Here we demonstrated that white-tailed deer (Odocoileus virginianus), an animal species in which the angiotensin converting enzyme 2 (ACE2) - the SARS-CoV-2 receptor - shares a high degree of similarity to humans, are highly susceptible to infection. Intranasal inoculation of deer fawns with SARS-CoV-2 resulted in established subclinical viral infection and shedding of infectious virus in nasal secretions. Notably, infected animals transmitted the virus to non-inoculated contact deer. Viral RNA was detected in multiple tissues 21 days post-inoculation (pi). All inoculated and indirect contact animals seroconverted and developed neutralizing antibodies as early as day 7 pi. The work provides important insights into the animal host range of SARS-CoV-2 and identifies white-tailed deer as a susceptible wild animal species to the virus.IMPORTANCEGiven the presumed zoonotic origin of SARS-CoV-2, the human-animal-environment interface of COVID-19 pandemic is an area of great scientific and public- and animal-health interest. Identification of animal species that are susceptible to infection by SARS-CoV-2 may help to elucidate the potential origin of the virus, identify potential reservoirs or intermediate hosts, and define the mechanisms underlying cross-species transmission to humans. Additionally, it may also provide information and help to prevent potential reverse zoonosis that could lead to the establishment of a new wildlife hosts. Our data show that upon intranasal inoculation, white-tailed deer became subclinically infected and shed infectious SARS-CoV-2 in nasal secretions and feces. Importantly, indirect contact animals were infected and shed infectious virus, indicating efficient SARS-CoV-2 transmission from inoculated animals. These findings support the inclusion of wild cervid species in investigations conducted to assess potential reservoirs or sources of SARS-CoV-2 of infection.
Figures

References
-
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL, Lauber C, Leontovich AM, Neuman BW, Penzar D, Perlman S, Poon LLM, Samborskiy DV, Sidorov IA, Sola I, Ziebuhr J. 2020. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol Nature Res 5:536–544. 10.1038/s41564-020-0695-z. - DOI - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

