Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells
- PMID: 33692342
- PMCID: PMC7946898
- DOI: 10.1038/s41413-020-00124-y
Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells
Abstract
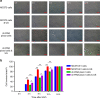
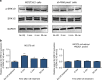
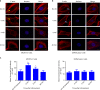
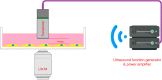
Mechanobiological stimuli, such as low-intensity pulsed ultrasound (LIPUS), have been shown to promote bone regeneration and fresh fracture repair, but the fundamental biophysical mechanisms involved remain elusive. Here, we propose that a mechanosensitive ion channel of Piezo1 plays a pivotal role in the noninvasive ultrasound-induced mechanical transduction pathway to trigger downstream cellular signal processes. This study aims to investigate the expression and role of Piezo1 in MC3T3-E1 cells after LIPUS treatment. Immunofluorescence analysis shows that Piezo1 was present on MC3T3-E1 cells and could be ablated by shRNA transfection. MC3T3-E1 cell migration and proliferation were significantly increased by LIPUS stimulation, and knockdown of Piezo1 restricted the increase in cell migration and proliferation. After labeling with Fluo-8, MC3T3-E1 cells exhibited fluorescence intensity traces with several high peaks compared with the baseline during LIPUS stimulation. No obvious change in the fluorescence intensity tendency was observed after LIPUS stimulation in shRNA-Piezo1 cells, which was similar to the results in the GsMTx4-treated group. The phosphorylation ratio of ERK1/2 in MC3T3-E1 cells was significantly increased (P < 0.01) after LIPUS stimulation. In addition, Phalloidin-iFluor-labeled F-actin filaments immediately accumulated in the perinuclear region after LIPUS stimulation, continued for 5 min, and then returned to their initial levels at 30 min. These results suggest that Piezo1 can transduce LIPUS-induced mechanical signals into intracellular calcium. The influx of Ca2+ serves as a second messenger to activate ERK1/2 phosphorylation and perinuclear F-actin filament polymerization, which regulate the proliferation of MC3T3-E1 cells.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hannemann PF, Mommers EH, Schots JP, Brink PR, Poeze M. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: a systematic review and meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 2014;134:1093–1106. doi: 10.1007/s00402-014-2014-8. - DOI - PubMed
Grants and funding
- R01 AR052379/AR/NIAMS NIH HHS/United States
- R01 AR061821/AR/NIAMS NIH HHS/United States
- AR052379/U.S. Department of Health & Human Services | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- AR061821/U.S. Department of Health & Human Services | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

