Development of a mugineic acid family phytosiderophore analog as an iron fertilizer
- PMID: 33692352
- PMCID: PMC7946895
- DOI: 10.1038/s41467-021-21837-6
Development of a mugineic acid family phytosiderophore analog as an iron fertilizer
Abstract
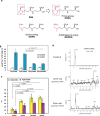
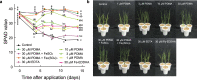
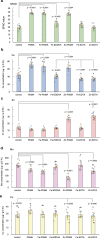
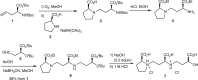
Iron (Fe) is an essential nutrient, but is poorly bioavailable because of its low solubility in alkaline soils; this leads to reduced agricultural productivity. To overcome this problem, we first showed that the soil application of synthetic 2'-deoxymugineic acid, a natural phytosiderophore from the Poaceae, can recover Fe deficiency in rice grown in calcareous soil. However, the high cost and poor stability of synthetic 2'-deoxymugineic acid preclude its agricultural use. In this work, we develop a more stable and less expensive analog, proline-2'-deoxymugineic acid, and demonstrate its practical synthesis and transport of its Fe-chelated form across the plasma membrane by Fe(III)•2'-deoxymugineic acid transporters. Possibility of its use as an iron fertilizer on alkaline soils is supported by promotion of rice growth in a calcareous soil by soil application of metal free proline-2'-deoxymugineic acid.
Conflict of interest statement
M.S. and A.M. are employed by the company AICHI STEEL CORPORATION. The remaining authors declare no competing interests.
Figures




References
-
- Wallace A, Lunt OR. Iron chlorosis in horticultural plants, a review. Proc. Am. Soc. Hortic. Sci. 1960;75:819–841.
-
- Vose PB. Iron nutrition in plants: a world overview. J. Plant Nutr. 1982;5:233–249. doi: 10.1080/01904168209362954. - DOI
-
- Takagi S. Naturally occurring iron-chelating compounds in oat-and rice-root washings: I. Activity measurement and preliminary characterization. Soil Sci. Plant. Nutr. 1976;22:423–433. doi: 10.1080/00380768.1976.10433004. - DOI
-
- Takemoto T, et al. Structure of mugineic acid, a new amino acid possessing an iron-chelating activity from roots washings of water-cultured hordeum vulgare L. Proc. Jpn Acad. 1978;54:469–473. doi: 10.2183/pjab.54.469. - DOI
-
- Takagi S. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 1984;7:469–477. doi: 10.1080/01904168409363213. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

