Channelling inflammation: gasdermins in physiology and disease
- PMID: 33692549
- PMCID: PMC7944254
- DOI: 10.1038/s41573-021-00154-z
Channelling inflammation: gasdermins in physiology and disease
Abstract
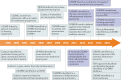
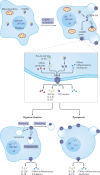
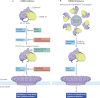
Gasdermins were recently identified as the mediators of pyroptosis - inflammatory cell death triggered by cytosolic sensing of invasive infection and danger signals. Upon activation, gasdermins form cell membrane pores, which release pro-inflammatory cytokines and alarmins and damage the integrity of the cell membrane. Roles for gasdermins in autoimmune and inflammatory diseases, infectious diseases, deafness and cancer are emerging, revealing potential novel therapeutic avenues. Here, we review current knowledge of the family of gasdermins, focusing on their mechanisms of action and roles in normal physiology and disease. Efforts to develop drugs to modulate gasdermin activity to reduce inflammation or activate more potent immune responses are highlighted.
Conflict of interest statement
H.W. and J.L. are co-founders of Ventus Therapeutics. The other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

