Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention
- PMID: 33692665
- PMCID: PMC7939128
- DOI: 10.1002/adfm.202006220
Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention
Abstract
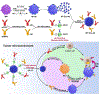
Macrophages are one of the most abundant non-malignant cells in the tumor microenvironment, playing critical roles in mediating tumor immunity. As important innate immune cells, macrophages possess the potential to engulf tumor cells and present tumor-specific antigens for adaptive antitumor immunity induction, leading to growing interest in targeting macrophage phagocytosis for cancer immunotherapy. Nevertheless, live tumor cells have evolved to evade phagocytosis by macrophages via the extensive expression of anti-phagocytic molecules, such as CD47. In addition, macrophages also rapidly recognize and engulf apoptotic cells (efferocytosis) in the tumor microenvironment, which inhibits inflammatory responses and facilitates immune escape of tumor cells. Thus, intervention of macrophage phagocytosis by blocking anti-phagocytic signals on live tumor cells or inhibiting tumor efferocytosis presents a promising strategy for the development of cancer immunotherapies. Here, the regulation of macrophage-mediated tumor cell phagocytosis is first summarized, followed by an overview of strategies targeting macrophage phagocytosis for the development of antitumor therapies. Given the potential off-target effects associated with the administration of traditional therapeutics (for example, monoclonal antibodies, small molecule inhibitors), we highlight the opportunity for nanomedicine in macrophage phagocytosis intervention.
Keywords: TAM receptors; cancer immunotherapy; efferocytosis; innate immune checkpoints; macrophage phagocytosis; nanomedicine.
Conflict of interest statement
Conflict of Interest LH is a consultant for PDS Biotechnology, Samyang Biopharmaceutical Co., Stemirna, and Beijing Inno Medicine. Other authors declare no conflict of interest.
Figures


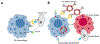



References
-
- De Palma M, Lewis CE, Cancer cell 2013, 23, 277; - PubMed
- DeNardo DG, Ruffell B, Nature reviews. Immunology 2019, 19, 369; - PMC - PubMed
- Ostuni R, Kratochvill F, Murray PJ, Natoli G, Trends in immunology 2015, 36, 229; - PubMed
- Pathria P, Louis TL, Varner JA, Trends in immunology 2019, 40, 310. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
