Relevance of the Pyroptosis-Related Inflammasome Pathway in the Pathogenesis of Diabetic Kidney Disease
- PMID: 33692782
- PMCID: PMC7937695
- DOI: 10.3389/fimmu.2021.603416
Relevance of the Pyroptosis-Related Inflammasome Pathway in the Pathogenesis of Diabetic Kidney Disease
Abstract
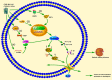
Diabetic kidney disease (DKD) is a major cause of chronic kidney disease (CKD) in many developed and developing countries. Pyroptosis is a recently discovered form of programmed cell death (PCD). With progress in research on DKD, researchers have become increasingly interested in elucidating the role of pyroptosis in DKD pathogenesis. This review focuses on the three pathways of pyroptosis generation: the canonical inflammasome, non-canonical inflammasome, and caspase-3-mediated inflammasome pathways. The molecular and pathophysiological mechanisms of the pyroptosis-related inflammasome pathway in the development of DKD are summarized. Activation of the diabetes-mediated pyroptosis-related inflammasomes, such as nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3), Toll-like receptor 4 (TLR4), caspase-1, interleukin (IL)-1β, and the IL-18 axis, plays an essential role in DKD lesions. By inhibiting activation of the TLR4 and NLRP3 inflammasomes, the production of caspase-1, IL-1β, and IL-18 is inhibited, thereby improving the pathological changes associated with DKD. Studies using high-glucose-induced cell models, high-fat diet/streptozotocin-induced DKD animal models, and human biopsies will help determine the spatial and temporal expression of DKD inflammatory components. Recent studies have confirmed the relationship between the pyroptosis-related inflammasome pathway and kidney disease. However, these studies are relatively superficial at present, and the mechanism needs further elucidation. Linking these findings with disease activity and prognosis would provide new ideas for DKD research.
Keywords: diabetic kidney disease; inflammasome pathway; pathogenesis; pyroptosis-related; targeted inhibition.
Copyright © 2021 Liu, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

