Do we need to improve the reporting of evidence in tendinopathy management? A critical appraisal of systematic reviews with recommendations on strength of evidence assessment
- PMID: 33692904
- PMCID: PMC7907875
- DOI: 10.1136/bmjsem-2020-000920
Do we need to improve the reporting of evidence in tendinopathy management? A critical appraisal of systematic reviews with recommendations on strength of evidence assessment
Abstract
Objective: To critically appraise the quality of published systematic reviews (SRs) of randomised controlled trials (RCTs) in tendinopathy with regard to handling and reporting of results with special emphasis on strength of evidence assessment.
Data sources: Medline from inception to June 2020.
Study eligibility: All SRs of RCTs assessing the effectiveness of any intervention(s) on any location of tendinopathy.
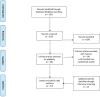
Data extraction and synthesis: Included SRs were appraised with the use of a 12-item tool devised by the authors arising from the Preferred Reporting Items in Systematic Reviews and Meta-Analyses statement and other relevant guidance. Subgroup analyses were performed based on impact factor (IF) of publishing journals and date of publication.
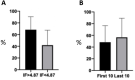
Results: A total of 57 SRs were included published in 38 journals between 2006 and 2020. The most commonly used risk-of-bias (RoB) assessment tool and strength of evidence assessment tool were the Cochrane Collaboration RoB tool and the Cochrane Collaboration Back Review Group tool, respectively. The mean score on the appraisal tool was 46.5% (range 0%-100%). SRs published in higher IF journals (>4.7) were associated with a higher mean score than those in lower IF journals (mean difference 26.4%±8.8%, p=0.004). The mean score of the 10 most recently published SRs was similar to that of the first 10 published SRs (mean difference 8.3%±13.7%, p=0.54). Only 23 SRs (40%) used the results of their RoB assessment in data synthesis and more than half (n=30; 50%) did not assess the strength of evidence of their results. Only 12 SRs (21%) assessed their strength of evidence appropriately.
Conclusions: In light of the poor presentation of evidence identified by our review, we provide recommendations to increase transparency and reproducibility in future SRs.
Keywords: evidence based review; tendinopathy; tendon.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Analysis of risk of bias assessments in a sample of intervention systematic reviews, Part II: focus on risk of bias tools reveals few meet current appraisal standards.J Clin Epidemiol. 2024 Oct;174:111460. doi: 10.1016/j.jclinepi.2024.111460. Epub 2024 Jul 16. J Clin Epidemiol. 2024. PMID: 39025376
-
Effectiveness and safety of manual therapy for knee osteoarthritis: An overview of systematic reviews and meta-analyses.Front Public Health. 2023 Feb 24;11:1081238. doi: 10.3389/fpubh.2023.1081238. eCollection 2023. Front Public Health. 2023. PMID: 36908468 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Analysis of risk of bias assessments in a sample of intervention systematic reviews, part I: many aspects of conduct and reporting need improvement.J Clin Epidemiol. 2024 Oct;174:111480. doi: 10.1016/j.jclinepi.2024.111480. Epub 2024 Jul 23. J Clin Epidemiol. 2024. PMID: 39047919
Cited by
-
Effect of resistance exercise dose components for tendinopathy management: a systematic review with meta-analysis.Br J Sports Med. 2023 Oct;57(20):1327-1334. doi: 10.1136/bjsports-2022-105754. Epub 2023 May 11. Br J Sports Med. 2023. PMID: 37169370 Free PMC article.
-
Exercise therapy for tendinopathy: a mixed-methods evidence synthesis exploring feasibility, acceptability and effectiveness.Health Technol Assess. 2023 Oct;27(24):1-389. doi: 10.3310/TFWS2748. Health Technol Assess. 2023. PMID: 37929629 Free PMC article.
References
-
- Cochrane . Cochrane consumer network. Available: https://consumers.cochrane.org/levels-evidence [Accessed 10 Jun 2020].
-
- van Tulder M, Furlan A, Bombardier C. Editorial board of the Cochrane collaboration back review group. updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 2003;28:1290–9. - PubMed
-
- Guyatt GH, Oxman AD, Vist GE, et al. . Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
