Comparative Efficacy and Safety of PARP Inhibitors as Maintenance Therapy in Platinum Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis
- PMID: 33692936
- PMCID: PMC7937863
- DOI: 10.3389/fonc.2020.573801
Comparative Efficacy and Safety of PARP Inhibitors as Maintenance Therapy in Platinum Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis
Abstract
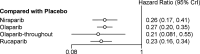
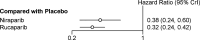
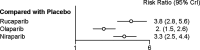
This meta-analysis investigated the comparative efficacy and safety of PARP inhibitor monotherapy as maintenance treatment in platinum sensitive recurrent ovarian cancer (ROC). Electronic databases were systematically searched for relevant RCTs. The primary endpoint was PFS. The results were stratified based on three categories: BRCA mutated patients, HRD patients, and overall population. The secondary outcome were discontinuations due to adverse events and grade 3 or 4 adverse events in maintenance phase. Five eligible RCTs were included in the network meta-analysis. For patients with BRCA mutated ovarian cancer, olaparib-throughout (HR = 0.21 with 95% CrI: 0.081-0.55), rucaparib (HR = 0.23 with 95% CrI: 0.16-0.34), olaparib (HR = 0.27 with 95% CrI: 0.20-0.35), and niraparib (HR = 0.26 with 95% CrI: 0.17-0.41) were all highly effective in comparison with placebo at improving PFS. For HRD patients, both rucaparib (HR = 0.32 with 95% CrI: 0.24-0.42) and niraparib (HR = 0.38 with 95% CrI: 0.24-0.60) were all highly effective in comparison with placebo at improving PFS. For the overall population, olaparib-throughout (HR = 0.51 with 95% CrI: 0.34-0.76), rucaparib (HR = 0.37 with 95% CrI: 0.30-0.45), olaparib (HR = 0.35 with 95% CrI: 0.25-0.49), and niraparib (HR = 0.38 with 95% CrI: 0.30-0.48) were all highly effective in comparison with placebo at improving PFS. Regarding grade 3 or 4 adverse events, the incidence of grade 3 or 4 toxicity reactions to rucaparib and niraparib were significantly higher than in the olaparib group. In terms of discontinuations due to adverse events, the treatment discontinuations were not significantly different between the three drugs. In summary, all the included maintenance treatment regimens are effective regardless of BRCA mutational status, and no statistically significant differences between rucaparib, niraparib and Olaparib in terms of PFS. In terms of safety profile, the three drugs present manageable adverse events. Clinicians should consider potential adverse events related to each of these interventions in clinical practice, and the adverse events are generally manageable.
Keywords: PARP inhibitor; network meta-analysis; ovarian cancer; platinum; progress-free survival.
Copyright © 2021 Xu, Ding, Tian, Bi, Han and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
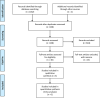


References
-
- Tomao F, Bardhi E, Di Pinto A, Sassu CM, Biagioli E, Petrella MC, et al. . Parp inhibitors as maintenance treatment in platinum sensitive recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials according to BRCA mutational status. Cancer Treat Rev (2019) 80:101909. 10.1016/j.ctrv.2019.101909 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

