Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection
- PMID: 33693030
- PMCID: PMC7937875
- DOI: 10.3389/fmolb.2021.632290
Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection
Abstract
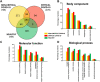
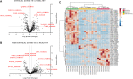
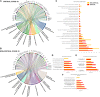
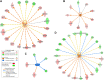
Knowledge of the host response to the novel coronavirus SARS-CoV-2 remains limited, hindering the understanding of COVID-19 pathogenesis and the development of therapeutic strategies. During the course of a viral infection, host cells release exosomes and other extracellular vesicles carrying viral and host components that can modulate the immune response. The present study used a shotgun proteomic approach to map the host circulating exosomes' response to SARS-CoV-2 infection. We investigated how SARS-CoV-2 infection modulates exosome content, exosomes' involvement in disease progression, and the potential use of plasma exosomes as biomarkers of disease severity. A proteomic analysis of patient-derived exosomes identified several molecules involved in the immune response, inflammation, and activation of the coagulation and complement pathways, which are the main mechanisms of COVID-19-associated tissue damage and multiple organ dysfunctions. In addition, several potential biomarkers-such as fibrinogen, fibronectin, complement C1r subcomponent and serum amyloid P-component-were shown to have a diagnostic feature presenting an area under the curve (AUC) of almost 1. Proteins correlating with disease severity were also detected. Moreover, for the first time, we identified the presence of SARS-CoV-2 RNA in the exosomal cargo, which suggests that the virus might use the endocytosis route to spread infection. Our findings indicate circulating exosomes' significant contribution to several processes-such as inflammation, coagulation, and immunomodulation-during SARS-CoV-2 infection. The study's data are available via ProteomeXchange with the identifier PXD021144.
Keywords: SARS-CoV-2; biomarkers; host-response; plasma exosomes; proteomics.
Copyright © 2021 Barberis, Vanella, Falasca, Caneapero, Cappellano, Raineri, Ghirimoldi, De Giorgis, Puricelli, Vaschetto, Sainaghi, Bruno, Sica, Dianzani, Rolla, Chiocchetti, Cantaluppi, Baldanzi, Marengo and Manfredi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Adamo A., Brandi J., Caligola S., Delfino P., Bazzoni R., Carusone R., et al. (2019). Extracellular vesicles mediate mesenchymal stromal cell-dependent regulation of B Cell PI3K-AKT signaling pathway and actin cytoskeleton. Front. Immunol. 10, 1664–3224. 10.3389/fimmu.2019.00446 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

