Advances in vaccine delivery systems against viral infectious diseases
- PMID: 33694083
- PMCID: PMC7945613
- DOI: 10.1007/s13346-021-00945-2
Advances in vaccine delivery systems against viral infectious diseases
Abstract
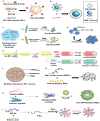
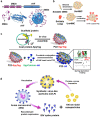
Although vaccines are available for many infectious diseases, there are still unresolved infectious diseases that threaten global public health. In particular, the rapid spread of unpredictable, highly contagious viruses has recorded numerous infection cases and deaths, and has changed our lives socially or economically through social distancing and wearing masks. The pandemics of unpredictable, highly contagious viruses increase the ever-high social need for rapid vaccine development. Nanotechnologies may hold promise and expedite the development of vaccines against newly emerging infectious viruses. As potential nanoplatforms for delivering antigens to immune cells, delivery systems based on lipids, polymers, proteins, and inorganic nanomaterials have been studied. These nanoplatforms have been tested as a means to deliver vaccines not as a whole, but in the form of protein subunits or as DNA or mRNA sequences encoding the antigen proteins of viruses. This review covers the current status of nanomaterial-based delivery systems for viral antigens, with highlights on nanovaccines against recently emerging infectious viruses, such as severe acute respiratory syndrome coronavirus-2, Middle East respiratory syndrome coronavirus, and Zika virus.
Keywords: Nanotechnology; Severe acute respiratory syndrome coronavirus-2; Vaccine delivery systems; Viral infectious diseases.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Minor PD. Live attenuated vaccines: Historical successes and current challenges. Virology. 2015;479–480:379–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

