The pro-inflammatory microRNA miR-155 influences fibrillar β-Amyloid1-42 catabolism by microglia
- PMID: 33694209
- PMCID: PMC9098129
- DOI: 10.1002/glia.23988
The pro-inflammatory microRNA miR-155 influences fibrillar β-Amyloid1-42 catabolism by microglia
Abstract
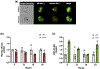
Microglia are the innate immune cells of the central nervous system that adopt rapid functional changes in response to Damage Associated Molecular Patterns, including aggregated β-Amyloid (Aβ) found in Alzheimer's disease (AD). microRNAs (miRNAs) are post-transcriptional modulators that influence the timing and magnitude of microglia inflammatory responses by downregulating the expression of inflammatory effectors. Recent studies implicate miR-155, a miRNA known to regulate inflammatory responses, in the pathogenesis of neurodegenerative disorders including multiple sclerosis, ALS, familial Parkinson's disease, and AD. In this work, we asked if miR-155 expression in microglia modifies cellular behaviors in response to fibrillar Aβ1-42 (fAβ1-42 ), in vitro. We hypothesized that in microglia, miR-155 expression would impact the internalization and catabolism of extracellular fAβ1-42 . Primary microglia stimulated with lipopolysaccharide demonstrate fast upregulation of miR-155 followed by delayed upregulation of miR-146a, an anti-inflammatory miRNA. Conditional overexpression of miR-155 in microglia resulted in significant upregulation of miR-146a. Conditional deletion of miR-155 promoted transit of fAβ1-42 to low-pH compartments where catabolism occurs, while miR-155 overexpression decreases fAβ1-42 catabolism. Uptake of fAβ1-42 across the plasma membrane increased with both up and downregulation of miR-155 expression. Taken together, our results support the hypothesis that inflammatory signaling influences the ability of microglia to catabolize fAβ1-42 through interconnected mechanisms modulated by miR-155. Understanding how miRNAs modulate the ability of microglia to catabolize fAβ1-42 will further elucidate the role of cellular players and molecular crosstalk in AD pathophysiology.
Keywords: catabolism; fibrillar Aβ1-42; miR-146a; miR-155; miRNAs; primary microglia.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflict of interest.
Figures
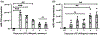




Similar articles
-
Lipopolysaccharide-Induced Exosomal miR-146a Is Involved in Altered Expression of Alzheimer's Risk Genes Via Suppression of TLR4 Signaling.J Mol Neurosci. 2021 Jun;71(6):1245-1255. doi: 10.1007/s12031-020-01750-1. Epub 2020 Nov 13. J Mol Neurosci. 2021. PMID: 33185814 Free PMC article.
-
MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer's disease.Theranostics. 2021 Feb 19;11(9):4103-4121. doi: 10.7150/thno.53418. eCollection 2021. Theranostics. 2021. PMID: 33754051 Free PMC article.
-
Microglia specific deletion of miR-155 in Alzheimer's disease mouse models reduces amyloid-β pathology but causes hyperexcitability and seizures.J Neuroinflammation. 2023 Mar 7;20(1):60. doi: 10.1186/s12974-023-02745-6. J Neuroinflammation. 2023. PMID: 36879321 Free PMC article.
-
Vesicular Transport of Encapsulated microRNA between Glial and Neuronal Cells.Int J Mol Sci. 2020 Jul 18;21(14):5078. doi: 10.3390/ijms21145078. Int J Mol Sci. 2020. PMID: 32708414 Free PMC article. Review.
-
The role of microglia in amyloid clearance from the AD brain.J Neural Transm (Vienna). 2010 Aug;117(8):949-60. doi: 10.1007/s00702-010-0433-4. Epub 2010 Jun 15. J Neural Transm (Vienna). 2010. PMID: 20552234 Free PMC article. Review.
Cited by
-
A cross-sectional study comparing the expression of DNA repair molecules in subjects with and without atherosclerotic plaques.Mol Biol Rep. 2024 Sep 4;51(1):953. doi: 10.1007/s11033-024-09886-8. Mol Biol Rep. 2024. PMID: 39230767
-
Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases.Transl Neurodegener. 2022 Dec 12;11(1):53. doi: 10.1186/s40035-022-00330-0. Transl Neurodegener. 2022. PMID: 36510311 Free PMC article. Review.
-
MiR-155: An Important Regulator of Neuroinflammation.Int J Mol Sci. 2021 Dec 22;23(1):90. doi: 10.3390/ijms23010090. Int J Mol Sci. 2021. PMID: 35008513 Free PMC article. Review.
-
Inflamma-MicroRNAs in Alzheimer's Disease: From Disease Pathogenesis to Therapeutic Potentials.Front Cell Neurosci. 2021 Oct 28;15:785433. doi: 10.3389/fncel.2021.785433. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34776873 Free PMC article. Review.
-
Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review.Int J Mol Sci. 2023 Feb 10;24(4):3574. doi: 10.3390/ijms24043574. Int J Mol Sci. 2023. PMID: 36834984 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

