Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19
- PMID: 33704352
- PMCID: PMC7953339
- DOI: 10.1001/jama.2021.3645
Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19
Abstract
Importance: Control of the global COVID-19 pandemic will require the development and deployment of safe and effective vaccines.
Objective: To evaluate the immunogenicity of the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson) in humans, including the kinetics, magnitude, and phenotype of SARS-CoV-2 spike-specific humoral and cellular immune responses.
Design, setting, and participants: Twenty-five participants were enrolled from July 29, 2020, to August 7, 2020, and the follow-up for this day 71 interim analysis was completed on October 3, 2020; follow-up to assess durability will continue for 2 years. This study was conducted at a single clinical site in Boston, Massachusetts, as part of a randomized, double-blind, placebo-controlled phase 1 clinical trial of Ad26.COV2.S.
Interventions: Participants were randomized to receive 1 or 2 intramuscular injections with 5 × 1010 viral particles or 1 × 1011 viral particles of Ad26.COV2.S vaccine or placebo administered on day 1 and day 57 (5 participants in each group).
Main outcomes and measures: Humoral immune responses included binding and neutralizing antibody responses at multiple time points following immunization. Cellular immune responses included immunospot-based and intracellular cytokine staining assays to measure T-cell responses.
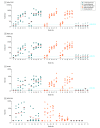
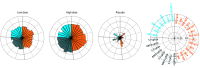
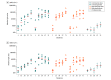
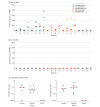
Results: Twenty-five participants were randomized (median age, 42; age range, 22-52; 52% women, 44% male, 4% undifferentiated), and all completed the trial through the day 71 interim end point. Binding and neutralizing antibodies emerged rapidly by day 8 after initial immunization in 90% and 25% of vaccine recipients, respectively. By day 57, binding and neutralizing antibodies were detected in 100% of vaccine recipients after a single immunization. On day 71, the geometric mean titers of spike-specific binding antibodies were 2432 to 5729 and the geometric mean titers of neutralizing antibodies were 242 to 449 in the vaccinated groups. A variety of antibody subclasses, Fc receptor binding properties, and antiviral functions were induced. CD4+ and CD8+ T-cell responses were induced.
Conclusion and relevance: In this phase 1 study, a single immunization with Ad26.COV2.S induced rapid binding and neutralization antibody responses as well as cellular immune responses. Two phase 3 clinical trials are currently underway to determine the efficacy of the Ad26.COV2.S vaccine.
Trial registration: ClinicalTrials.gov Identifier: NCT04436276.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

