Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer
- PMID: 33704353
- PMCID: PMC7953335
- DOI: 10.1001/jamaoncol.2021.0057
Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer
Abstract
Importance: Surgical resection has been considered the only curative option for patients with pancreatic cancer. Nonoperative local treatment options that can provide a similar benefit are needed. Emerging radiation techniques that address organ motion have enabled curative radiation doses to be given in patients with inoperable disease.
Objective: To determine the association of hypofractionated ablative radiation therapy (A-RT) with survival for patients with locally advanced pancreatic cancer (LAPC) treated with a novel radiation planning and delivery technique.
Design, setting, and participants: This cohort study included 119 consecutive patients treated with A-RT between June 2016 and February 2019 and enrolled in a prospectively maintained database. Patients were treated with a standardized technique within a large academic cancer center regional network. All patients with localized, unresectable, or medically inoperable pancreatic cancer with tumors of any size and less than 5 cm luminal abutment with the primary tumor were eligible.
Interventions: Ablative RT (98 Gy biologically effective dose) was delivered using standard equipment. Respiratory gating, soft tissue image guidance, and selective adaptive planning were used to address organ motion and limit the dose to surrounding luminal organs.
Main outcomes and measures: The primary outcome was overall survival (OS). Secondary outcomes included incidence of local progression and progression-free survival.
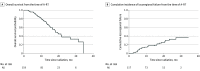
Results: Between 2016 and 2019, 119 patients (59 men, median age 67 years) received A-RT, including 99 with T3/T4 and 53 with node-positive disease, with a median carbohydrate antigen 19-9 (CA19-9) level greater than 167 U/mL. Most (116 [97.5%]) received induction chemotherapy for a median of 4 months (0.5-18.4). Median OS from diagnosis and A-RT were 26.8 and 18.4 months, respectively. Respective 12- and 24-month OS from A-RT were 74% (95% CI, 66%-83%) and 38% (95% CI, 27%-52%). Twelve- and 24-month cumulative incidence of locoregional failure were 17.6% (95% CI, 10.4%-24.9%) and 32.8% (95% CI, 21.6%-44.1%), respectively. Postinduction CA19-9 decline was associated with improved locoregional control and survival. Grade 3 upper gastrointestinal bleeding occurred in 10 patients (8%) with no grade 4 to 5 events.
Conclusions and relevance: This cohort study of patients with inoperable LAPC found that A-RT following multiagent induction therapy for LAPC was associated with durable locoregional tumor control and favorable survival. Prospective randomized trials in patients with LAPC are warranted.
Conflict of interest statement
Figures

Comment in
-
Ablative Radiotherapy for Patients With Inoperable Pancreas Cancer-Ready for Prime Time?JAMA Oncol. 2021 May 1;7(5):687-688. doi: 10.1001/jamaoncol.2021.0028. JAMA Oncol. 2021. PMID: 33704355 No abstract available.
References
-
- Hammel P, Huguet F, van Laethem JL, et al. ; LAP07 Trial Group . Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315(17):1844-1853. doi:10.1001/jama.2016.4324 - DOI - PubMed
-
- Chauffert B, Mornex F, Bonnetain F, et al. . Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592-1599. doi:10.1093/annonc/mdn281 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

