Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening
- PMID: 33704435
- PMCID: PMC7989519
- DOI: 10.1093/cid/ciab229
Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening
Abstract
Background: Several vaccines are now available under emergency use authorization in the United States and have demonstrated efficacy against symptomatic COVID-19. Vaccine impact on asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is largely unknown.
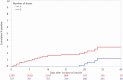
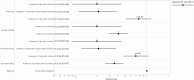
Methods: We conducted a retrospective cohort study of consecutive, asymptomatic adult patients (n = 39 156) within a large US healthcare system who underwent 48 333 preprocedural SARS-CoV-2 molecular screening tests between 17 December 2020 and 8 February 2021. The primary exposure of interest was vaccination with ≥1 dose of an mRNA COVID-19 vaccine. The primary outcome was relative risk (RR) of a positive SARS-CoV-2 molecular test among those asymptomatic persons who had received ≥1 dose of vaccine compared with persons who had not received vaccine during the same time period. RR was adjusted for age, sex, race/ethnicity, patient residence relative to the hospital (local vs nonlocal), healthcare system regions, and repeated screenings among patients using mixed-effects log-binomial regression.
Results: Positive molecular tests in asymptomatic individuals were reported in 42 (1.4%) of 3006 tests and 1436 (3.2%) of 45 327 tests performed on vaccinated and unvaccinated patients, respectively (RR, .44; 95% CI, .33-.60; P < .0001). Compared with unvaccinated patients, risk of asymptomatic SARS-CoV-2 infection was lower among those >10 days after the first dose (RR, .21; 95% CI, .12-.37; P < .0001) and >0 days after the second dose (RR, .20; 95% CI, .09-.44; P < .0001) in the adjusted analysis.
Conclusions: COVID-19 vaccination with an mRNA-based vaccine showed a significant association with reduced risk of asymptomatic SARS-CoV-2 infection as measured during preprocedural molecular screening. Results of this study demonstrate the impact of the vaccines on reduction in asymptomatic infections supplementing the randomized trial results on symptomatic patients.
Keywords: COVID-19; SARS-CoV-2; asymptomatic; vaccination.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Coronavirus disease (COVID-19) dashboard. Geneva, Switzerland; World Health Organization. Available at: https://covid19.who.int/. Accessed 12 February 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

