Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening
- PMID: 33704435
- PMCID: PMC7989519
- DOI: 10.1093/cid/ciab229
Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening
Abstract
Background: Several vaccines are now available under emergency use authorization in the United States and have demonstrated efficacy against symptomatic COVID-19. Vaccine impact on asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is largely unknown.
Methods: We conducted a retrospective cohort study of consecutive, asymptomatic adult patients (n = 39 156) within a large US healthcare system who underwent 48 333 preprocedural SARS-CoV-2 molecular screening tests between 17 December 2020 and 8 February 2021. The primary exposure of interest was vaccination with ≥1 dose of an mRNA COVID-19 vaccine. The primary outcome was relative risk (RR) of a positive SARS-CoV-2 molecular test among those asymptomatic persons who had received ≥1 dose of vaccine compared with persons who had not received vaccine during the same time period. RR was adjusted for age, sex, race/ethnicity, patient residence relative to the hospital (local vs nonlocal), healthcare system regions, and repeated screenings among patients using mixed-effects log-binomial regression.
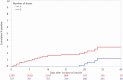
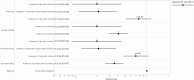
Results: Positive molecular tests in asymptomatic individuals were reported in 42 (1.4%) of 3006 tests and 1436 (3.2%) of 45 327 tests performed on vaccinated and unvaccinated patients, respectively (RR, .44; 95% CI, .33-.60; P < .0001). Compared with unvaccinated patients, risk of asymptomatic SARS-CoV-2 infection was lower among those >10 days after the first dose (RR, .21; 95% CI, .12-.37; P < .0001) and >0 days after the second dose (RR, .20; 95% CI, .09-.44; P < .0001) in the adjusted analysis.
Conclusions: COVID-19 vaccination with an mRNA-based vaccine showed a significant association with reduced risk of asymptomatic SARS-CoV-2 infection as measured during preprocedural molecular screening. Results of this study demonstrate the impact of the vaccines on reduction in asymptomatic infections supplementing the randomized trial results on symptomatic patients.
Keywords: COVID-19; SARS-CoV-2; asymptomatic; vaccination.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Association Between Vaccination With BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers.JAMA. 2021 Jun 22;325(24):2457-2465. doi: 10.1001/jama.2021.7152. JAMA. 2021. PMID: 33956048 Free PMC article.
-
Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data.Lancet. 2021 May 15;397(10287):1819-1829. doi: 10.1016/S0140-6736(21)00947-8. Epub 2021 May 5. Lancet. 2021. PMID: 33964222 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients With Cancer: A US Nationwide Veterans Affairs Study.JAMA Oncol. 2022 Feb 1;8(2):281-286. doi: 10.1001/jamaoncol.2021.5771. JAMA Oncol. 2022. PMID: 34854921 Free PMC article.
-
Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis.Lancet Child Adolesc Health. 2023 Jun;7(6):379-391. doi: 10.1016/S2352-4642(23)00078-0. Epub 2023 Apr 18. Lancet Child Adolesc Health. 2023. PMID: 37084750 Free PMC article.
Cited by
-
COVID-19 Vaccine Effectiveness: A Review of the First 6 Months of COVID-19 Vaccine Availability (1 January-30 June 2021).Vaccines (Basel). 2022 Mar 3;10(3):393. doi: 10.3390/vaccines10030393. Vaccines (Basel). 2022. PMID: 35335025 Free PMC article. Review.
-
Immunologic features of asymptomatic postvaccination infections with the Delta variant of SARS-CoV-2 in adults.Immun Inflamm Dis. 2022 Jul;10(7):e670. doi: 10.1002/iid3.670. Immun Inflamm Dis. 2022. PMID: 35759224 Free PMC article.
-
Indirect Protection by Reducing Transmission: Ending the Pandemic With Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination.Open Forum Infect Dis. 2021 May 19;9(2):ofab259. doi: 10.1093/ofid/ofab259. eCollection 2022 Feb. Open Forum Infect Dis. 2021. PMID: 35071679 Free PMC article. No abstract available.
-
Prioritizing high-contact occupations raises effectiveness of vaccination campaigns.Sci Rep. 2022 Jan 14;12(1):737. doi: 10.1038/s41598-021-04428-9. Sci Rep. 2022. PMID: 35031651 Free PMC article.
-
Vaccine Effects on Susceptibility and Symptomatology Can Change the Optimal Allocation of COVID-19 Vaccines: South Korea as an Example.J Clin Med. 2021 Jun 25;10(13):2813. doi: 10.3390/jcm10132813. J Clin Med. 2021. PMID: 34202324 Free PMC article.
References
-
- World Health Organization. Coronavirus disease (COVID-19) dashboard. Geneva, Switzerland; World Health Organization. Available at: https://covid19.who.int/. Accessed 12 February 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

