Considering Sex as a Biological Variable in Basic and Clinical Studies: An Endocrine Society Scientific Statement
- PMID: 33704446
- PMCID: PMC8348944
- DOI: 10.1210/endrev/bnaa034
Considering Sex as a Biological Variable in Basic and Clinical Studies: An Endocrine Society Scientific Statement
Abstract
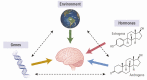
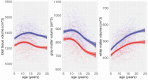
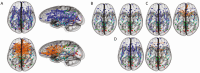
In May 2014, the National Institutes of Health (NIH) stated its intent to "require applicants to consider sex as a biological variable (SABV) in the design and analysis of NIH-funded research involving animals and cells." Since then, proposed research plans that include animals routinely state that both sexes/genders will be used; however, in many instances, researchers and reviewers are at a loss about the issue of sex differences. Moreover, the terms sex and gender are used interchangeably by many researchers, further complicating the issue. In addition, the sex or gender of the researcher might influence study outcomes, especially those concerning behavioral studies, in both animals and humans. The act of observation may change the outcome (the "observer effect") and any experimental manipulation, no matter how well-controlled, is subject to it. This is nowhere more applicable than in physiology and behavior. The sex of established cultured cell lines is another issue, in addition to aneuploidy; chromosomal numbers can change as cells are passaged. Additionally, culture medium contains steroids, growth hormone, and insulin that might influence expression of various genes. These issues often are not taken into account, determined, or even considered. Issues pertaining to the "sex" of cultured cells are beyond the scope of this Statement. However, we will discuss the factors that influence sex and gender in both basic research (that using animal models) and clinical research (that involving human subjects), as well as in some areas of science where sex differences are routinely studied. Sex differences in baseline physiology and associated mechanisms form the foundation for understanding sex differences in diseases pathology, treatments, and outcomes. The purpose of this Statement is to highlight lessons learned, caveats, and what to consider when evaluating data pertaining to sex differences, using 3 areas of research as examples; it is not intended to serve as a guideline for research design.
Keywords: brain-gut; cardiovascular disease; chromosome complement; gender; sex differences; steroid hormones.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Baclawski K. The Observer Effect . 2018 IEEE Conference on Cognitive and Computational Aspects of Situation Management (CogSIMA). Boston, MA, 2018:83-89. doi:10.1109/COGSIMA.2018.8423983.
-
- van Anders SM, Goldey KL, Kuo PX. The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36(9):1265-1275. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

