Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis
- PMID: 33704781
- PMCID: PMC8407366
- DOI: 10.1002/14651858.CD013306.pub2
Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis
Abstract
Background: Vascular cognitive impairment (VCI) describes a broad spectrum of cognitive impairments caused by cerebrovascular disease, ranging from mild cognitive impairment to dementia. There are currently no pharmacological treatments recommended for improving either cognition or function in people with VCI. Three cholinesterase inhibitors (donepezil, galantamine, and rivastigmine) are licenced for the treatment of dementia due to Alzheimer's disease. They are thought to work by compensating for reduced cholinergic neurotransmission, which is also a feature of VCI. Through pairwise comparisons with placebo and a network meta-analysis, we sought to determine whether these medications are effective in VCI and whether there are differences between them with regard to efficacy or adverse events.
Objectives: (1) To assess the efficacy and safety of cholinesterase inhibitors in the treatment of adults with vascular dementia and other VCI. (2) To compare the effects of different cholinesterase inhibitors on cognition and adverse events, using network meta-analysis.
Search methods: We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group's register, MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), Web of Science Core Collection (ISI Web of Science), LILACS (BIREME), ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform on 19 August 2020.
Selection criteria: We included randomised controlled trials in which donepezil, galantamine, or rivastigmine was compared with placebo or in which the drugs were compared with each other in adults with vascular dementia or other VCI (excluding cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)). We included all drug doses and routes of administration.
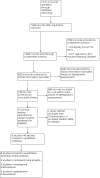
Data collection and analysis: Two review authors independently identified eligible trials, extracted data, assessed risk of bias, and applied the GRADE approach to assess the certainty of the evidence. The primary outcomes were cognition, clinical global impression, function (performance of activities of daily living), and adverse events. Secondary outcomes were serious adverse events, incidence of development of new dementia, behavioural disturbance, carer burden, institutionalisation, quality of life and death. For the pairwise analyses, we pooled outcome data at similar time points using random-effects methods. We also performed a network meta-analysis using Bayesian methods.
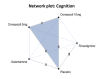
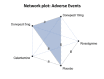
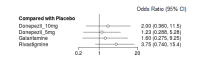
Main results: We included eight trials (4373 participants) in the review. Three trials studied donepezil 5 mg or 10 mg daily (n= 2193); three trials studied rivastigmine at a maximum daily dose of 3 to 12 mg (n= 800); and two trials studied galantamine at a maximum daily dose of 16 to 24 mg (n= 1380). The trials included participants with possible or probable vascular dementia or cognitive impairment following stroke. Mean ages were between 72.2 and 73.9 years. All of the trials were at low or unclear risk of bias in all domains, and the evidence ranged from very low to high level of certainty. For cognition, the results showed that donepezil 5 mg improves cognition slightly, although the size of the effect is unlikely to be clinically important (mean difference (MD) -0.92 Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) points (range 0 to 70), 95% confidence interval (CI) -1.44 to -0.40; high-certainty evidence). Donepezil 10 mg (MD -2.21 ADAS-Cog points, 95% CI -3.07 to -1.35; moderate-certainty evidence) and galantamine 16 to 24 mg (MD -2.01 ADAS-Cog point, 95%CI -3.18 to -0.85; moderate-certainty evidence) probably also improve cognition, although the larger effect estimates still may not be clinically important. With low certainty, there may be little to no effect of rivastigmine 3 to 12 mg daily on cognition (MD 0.03 ADAS-Cog points, 95% CI -3.04 to 3.10; low-certainty evidence). Adverse events reported in the studies included nausea and/or vomiting, diarrhoea, dizziness, headache, and hypertension. The results showed that there was probably little to no difference between donepezil 5 mg and placebo in the number of adverse events (odds ratio (OR) 1.22, 95% CI 0.94 to 1.58; moderate-certainty evidence), but there were slightly more adverse events with donepezil 10 mg than with placebo (OR 1.95, 95% CI 1.20 to 3.15; high-certainty evidence). The effect of rivastigmine 3 to 12 mg on adverse events was very uncertain (OR 3.21, 95% CI 0.36 to 28.88; very low-certainty evidence). Galantamine 16 to 24 mg is probably associated with a slight excess of adverse events over placebo (OR 1.57, 95% CI 1.02 to 2.43; moderate-certainty evidence). In the network meta-analysis (NMA), we included cognition to represent benefit, and adverse events to represent harm. All drugs ranked above placebo for cognition and below placebo for adverse events. We found donepezil 10 mg to rank first in terms of benefit, but third in terms of harms, when considering the network estimates and quality of evidence. Galantamine was ranked second in terms of both benefit and harm. Rivastigmine had the lowest ranking of the cholinesterase inhibitors in both benefit and harm NMA estimates, but this may reflect possibly inadequate doses received by some trial participants and small trial sample sizes.
Authors' conclusions: We found moderate- to high-certainty evidence that donepezil 5 mg, donepezil 10 mg, and galantamine have a slight beneficial effect on cognition in people with VCI, although the size of the change is unlikely to be clinically important. Donepezil 10 mg and galantamine 16 to 24 mg are probably associated with more adverse events than placebo. The evidence for rivastigmine was less certain. The data suggest that donepezil 10 mg has the greatest effect on cognition, but at the cost of adverse effects. The effect is modest, but in the absence of any other treatments, people living with VCI may still wish to consider the use of these agents. Further research into rivastigmine is needed, including the use of transdermal patches.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Ceri E Battle: none known
Azmil H Abdul‐Rahim: none known
Susan D Shenkin: none known
Jonathan Hewitt: none known
Terry J Quinn: none known
Figures







































Update of
References
References to studies included in this review
Auchus 2007 {published data only}
-
- Auchus A. A randomized, 26-week, double-blind, placebo-controlled trial to evaluate the safety and efficacy of galantamine in the treatment of dementia secondary to cerebrovascular disease. IFPMA register 2004;1:1-3.
-
- Auchus AP, Brashear HR, Salloway S, Korczyn AD, De Deyn PP, Gassmann-Mayer C, for the GAL-INT-26 Study Group. Galantamine treatment of vascular dementia: a randomised trial. Neurology 2007;69:448-58. - PubMed
-
- NCT00035191. A placebo controlled trial to evaluate the safety and efficacy of galantamine in the treatment of vascular dementia [A randomized 26 week double blind placebo controlled trial to evaluate the safety and efficacy of galantamine in the treatment of vascular dementia]. https://clinicaltrials.gov/ct2/show/NCT00035191 First received: 03 May 2002.
Ballard 2008 {published data only}
-
- Ballard C, Sauter M, Scheltens P, He Y, Barkhof F, Van Straaten ECW, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the Vantage study. Current Medical Research and Opinion 2008;24(9):2561-74. [DOI: 10.1185/03007990802328142] - DOI - PubMed
Black 2003 {published data only}
-
- Black S, Roman GC, Geldmacker DS, Salloway S, Hecker J, Burns A, et al, the Donepezil 307 Vascular Dementia Study Group. Efficacy and tolerability of Donepezil in vascular dementia: positive results of a 24-week, multi-centre, international, randomised, placebo-controlled trial. Stroke 2003;34:2323-32. [DOI: 10.1161/01.STR.0000091396.95360.E1] - DOI - PubMed
-
- Doody R, Pratt RD, Posner H, Kumar D. Vascular dementia patients who receive Donepezil treatment for 12 to 18 months maintain cognitive benefits. Neurobiology of Aging 2004;25:469.
-
- Geldmacher DS, Pratt PD, Perdomo CA. Donepezil slows functional deterioration in patients with vascular dementia. European Journal of Neurology 2002;9:35.
-
- Pratt D. Patient populations in clinical trials of the efficacy and tolerability of donepezil in patients with vascular dementia. Journal of the Neurological Sciences 2002;203:57-65. - PubMed
-
- Pratt R, Perdomo C, The Donepezil 307 and 308 Study Groups. Donepezil is well tolerated in patients with vascular dementia: a comparison of safety and tolerability results from randomized, placebo-controlled clinical trials in vascular dementia patients and Alzheimer's disease patients. Neurobiology of Aging 2002;23(1 Suppl S57):S57.
Erkinjuntti 2002 {published data only}
-
- Bullock R, Erkinjuntti T, Lilienfeld S, GAL-INT-6 Study Group. Management of patients with Alzheimer's disease plus cerebrovascular disease: 12-month treatment with galantamine. Dementia and Geriatric Cognitive Disorders 2004;17:29-34. - PubMed
-
- Burke W, Lilienfeld S. Galantamine improves behaviour and relieves caregiver distress in Alzheimer's disease (AD), vascular dementia and AD with cerebrovascular disease. In: 8th international conference on Alzheimer's disease and related disorders; 2002 Jul 20-25; Stockholm. 2002.
-
- Erkinjuntti T, Gauthier S, Bullock R, Kurz A, Hammond G, Schwalen S, et al. Galantamine treatment in Alzheimer's disease with cerebrovascular disease: responder analyses from a randomized, controlled trial (GAL-INT-6). Journal of Psychopharmacology 2008;22(7):761-8. - PubMed
-
- Erkinjuntti T, Kurz A, Gauthier A, Bullock R, Lillienfeld S, Damaraju CV. Efficacy of Galantamine in probable vascular dementia and Alzheimer's disease combined with cardiovascular disease: a randomised trial. Lancet 2002;359:1283-90. - PubMed
-
- Erkinjuntti T, Kurz A, Small GW, Bullock R, Lilienfeld S, Damaraju CV, GAL-INT-6 Study Group. An open-label extension trial of galantamine in patients with probable vascular dementia and mixed dementia. Clinical Therapeutics 2003;25:1765-82. - PubMed
Mok 2007 {published data only}
Narasimhalu 2009 {published data only}
-
- Narasimhalu K, Effendy S, Sim CH, Lee JM, Chen I, Hia SB, et al. A randomised controlled trial of Rivastigmine in patients with cognitive impairment no dementia because of cerebrovascular disease. Acta Neurologica Scandinavica 2010;121:217-24. - PubMed
Roman 2010 {published data only}
-
- Pratt RD, Perdomo CA. Donepezil improves cognitive function in patients with vascular dementia: results from study 307, a 24-week, randomized, double-blind, placebo-controlled trial. Portal of Geriatrics Online Education 2002;7:A161-2.
Wilkinson 2003 {published data only}
-
- Aguilar M, Roman G, Black S, Royall D, Surick I, Kumar D, et al. Efficacy and safety of donepezil in vascular dementia results from largest double-blind trial in vascular dementia patients. In: 10th International Conference on Alzheimer's Disease and Related Disorders; 2006 Jul 15-20; Madrid. 2006.
-
- Bayer A, Pratt RD, Kumar D. A comparison of the cognitive benefits of donepezil in patients with cortical and subcortical vascular dementia. In: 8th Congress of the European Federation of the Neurological Sciences; 2004 Sep 04-07; Paris. 2004.
-
- Boundy K, Pratt R. Donepezil provides significant benefits in patients with vascular dementia. Internal Medicine Journal 2003;33:A46.
-
- Farlow M. Efficacy of donepezil in vascular dementia. Neurology 2003;61(4):429. - PubMed
-
- Pratt R, Perdomo C. Donepezil is well tolerated in patients with vascular dementia: a comparison of safety and tolerability results from randomized, placebo-controlled clinical trials in vascular dementia patients and Alzheimer's disease patients. Neurobiology of Aging 2002;1:S57.
References to studies excluded from this review
Dichgans 2008 {published data only}
-
- Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurology 2008;7:310-8. - PubMed
Additional references
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Society, 2013.
Birks 2006
Birks 2013
Chen 2016
-
- Chen Y, Zhang J, Wang Y, Yuan J, Hu W. Efficacy of cholinesterase inhibitors in vascular dementia: an updated meta-analysis. European Neurology 2016;75:132-41. - PubMed
Colović 2013
Craig 2006
Dawbarn 2001
-
- Dawbarn D, Shelly JA. Neurobiology of Alzheimer’s Disease. 2nd edition. Oxford (UK): Oxford University Press, 2001.
Dichgans 2017
Erkinjuntti 2004
-
- Erkinjuntti T, Román G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke 2004;35:1010-7. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 12 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2008. Available at gradepro.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Hort 2010
-
- Hort J, O’Brien JT, Gainotti G, Pirttila T, Popescu BO, Rektorova I, et al. EFNS guidelines for the diagnosis and management of Alzheimer’s disease. European Journal of Neurology 2010;17:1236-48. - PubMed
Jackson 2014
Kavirajan 2007
-
- Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurology 2007;6:782-92. - PubMed
Kibret 2014
Li 2015
Lilienfeld 2002
Malouf 2004
Manero 2013
-
- Manero RM, Casals-Coll M, Sánchez-Benavides G, Rodríguez-de los Reyes ON, Aguilar M, Badenes D, et al, for the NEURONORMA Study Team. Diagnostic validity of the Alzheimer’s Disease Functional Assessment and Change Scale in mild cognitive impairment and mild to moderate Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders 2013;37(5):366-75. [DOI: 10.1159/000350800] - DOI - PubMed
Marshall 2018
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 Sep 13-16; Cape Town. 2017.
Mills 2013
NICE 2018
-
- National Institute for Health and Care Excellence. NICE guidelines: donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease. Technology appraisal guidance [TA217]. www.nice.org.uk/guidance/ta217 (accessed 6 January 2019).
Noel‐Storr 2018
-
- Noel-Storr AH, Project Transform Team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 Jun 18-20; Oxford. 2018.
O'Brien 2003
-
- O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet Neurology 2003;2:89-98. - PubMed
Owen 2019
Perry 1997
-
- Perry E, Kay DW. Some developments in brain ageing and dementia. British Journal of Biomedical Science 1997;54:201-15. - PubMed
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. - PubMed
Qiu 2009
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ritter 2015
Roman 1993
-
- Roman G, Tatemichi T, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993;43:250-60. - PubMed
Rücker 2015
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163-71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3:80-97. - PubMed
Schrag 2012
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Skrobot 2017
-
- Skrobot OA, O’Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s & Dementia 2017;13:624-33. - PubMed
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living Systematic Review Network. Living Systematic Reviews: 2. Combining Human and Machine Effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Toghi 1996
-
- Toghi H, Abe T, Kimura M, Saheki M, Takahashi S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarcts types as compared with Alzheimer-type dementia. Journal of Neural Transmission 1996;103:1211-20. - PubMed
Turner 2012
van der Flier 2018
-
- Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CL, et al. Vascular cognitive impairment. Nature Reviews Disease Primers 2018;15:18003. - PubMed
Veroniki 2013
WHO 1992
-
- World Health Organization. The ICD-11 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostic Guidelines. 10th edition. Geneva: World Health Organization, 1992.
Wortmann 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

