Chemically Fueled Self-Assembly in Biology and Chemistry
- PMID: 33704885
- PMCID: PMC8453758
- DOI: 10.1002/anie.202100274
Chemically Fueled Self-Assembly in Biology and Chemistry
Abstract
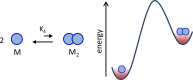
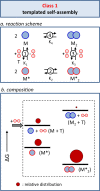
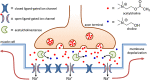
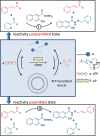
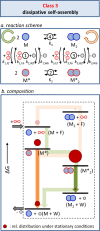
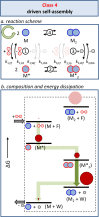
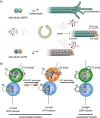
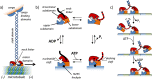
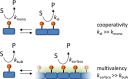
Life is a non-equilibrium state of matter maintained at the expense of energy. Nature uses predominantly chemical energy stored in thermodynamically activated, but kinetically stable, molecules. These high-energy molecules are exploited for the synthesis of other biomolecules, for the activation of biological machinery such as pumps and motors, and for the maintenance of structural order. Knowledge of how chemical energy is transferred to biochemical processes is essential for the development of artificial systems with life-like processes. Here, we discuss how chemical energy can be used to control the structural organization of organic molecules. Four different strategies have been identified according to a distinguishable physical-organic basis. For each class, one example from biology and one from chemistry are discussed in detail to illustrate the practical implementation of each concept and the distinct opportunities they offer. Specific attention is paid to the discussion of chemically fueled non-equilibrium self-assembly. We discuss the meaning of non-equilibrium self-assembly, its kinetic origin, and strategies to develop synthetic non-equilibrium systems.
Keywords: chemical fuel; non-equilibrium systems; origin of life; self-assembly; systems chemistry.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Schrödinger E., What Is Life? The Physical Aspect of the Living Cell, Cambridge Univ Press, Cambridge, 1944.
-
- Onsager L., Phys. Rev. 1931, 37, 405–426.
-
- Nicolis G., Prigogine I., Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order through Fluctuations, Wiley, New York, 1977.
-
- Adamski P., Eleveld M., Sood A., Kun Á., Szilágyi A., Czárán T., Szathmáry E., Otto S., Nat. Rev. Chem. 2020, 4, 386–403. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

