Physiologically-based pharmacokinetic modeling to predict drug interactions of lemborexant with CYP3A inhibitors
- PMID: 33704920
- PMCID: PMC8129715
- DOI: 10.1002/psp4.12606
Physiologically-based pharmacokinetic modeling to predict drug interactions of lemborexant with CYP3A inhibitors
Abstract
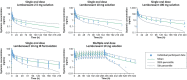
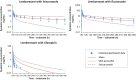
Lemborexant, a recently approved dual orexin receptor antagonist for treatment of adults with insomnia, is eliminated primarily by cytochrome P450 (CYP)3A metabolism. The recommended dose of lemborexant is 5 mg once per night, with a maximum recommended dose of 10 mg once daily. A physiologically-based pharmacokinetic (PBPK) model for lemborexant was developed and applied to integrate data obtained from in vivo drug-drug interaction (DDI) assessments, and to further explore lemborexant interaction with CYP3A inhibitors and inducers. The model predictions were in good agreement with observed pharmacokinetic data and with DDI results from clinical studies with CYP3A inhibitors, itraconazole and fluconazole. The model further predicted that DDI effects of weak CYP3A inhibitors (fluoxetine and ranitidine) are weak, and effects of moderate inhibitors (erythromycin and verapamil) are moderate. Based on the PBPK simulations and clinical efficacy and safety data, the maximum daily recommended lemborexant dose when administered with weak CYP3A inhibitors is 5 mg; co-administration of moderate and strong inhibitors should be avoided except in countries where 2.5 mg has been approved.
© 2021 Eisai Inc. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
T.U. and Y.M. are employees of Eisai Co. Ltd., Japan. I.L., B.L., and E.S. are employees of Eisai Inc., USA.
Figures
Similar articles
-
Evaluation of the CYP3A and CYP2B6 Drug-Drug Interaction Potential of Lemborexant.Clin Pharmacol Drug Dev. 2021 Jun;10(6):681-690. doi: 10.1002/cpdd.915. Epub 2021 Jan 17. Clin Pharmacol Drug Dev. 2021. PMID: 33455055 Free PMC article. Clinical Trial.
-
Physiologically based pharmacokinetic modeling and simulation to predict drug-drug interactions of ivosidenib with CYP3A perpetrators in patients with acute myeloid leukemia.Cancer Chemother Pharmacol. 2020 Nov;86(5):619-632. doi: 10.1007/s00280-020-04148-3. Epub 2020 Sep 25. Cancer Chemother Pharmacol. 2020. PMID: 32978634
-
Physiologically Based Pharmacokinetic Modeling of Palbociclib.J Clin Pharmacol. 2017 Feb;57(2):173-184. doi: 10.1002/jcph.792. Epub 2016 Aug 22. J Clin Pharmacol. 2017. PMID: 27402157 Clinical Trial.
-
A Comprehensive Review of Lemborexant to Treat Insomnia.Psychopharmacol Bull. 2024 Mar 4;54(1):43-64. Psychopharmacol Bull. 2024. PMID: 38449475 Free PMC article. Review.
-
Review of the Efficacy and Safety of Lemborexant, a Dual Receptor Orexin Antagonist (DORA), in the Treatment of Adults With Insomnia Disorder.Ann Pharmacother. 2022 Feb;56(2):213-221. doi: 10.1177/10600280211008492. Epub 2021 Jun 2. Ann Pharmacother. 2022. PMID: 34078141 Review.
Cited by
-
Projection of Target Drug Particle Size in Oral Formulations Using the Refined Developability Classification System (rDCS).Pharmaceutics. 2023 Jul 8;15(7):1909. doi: 10.3390/pharmaceutics15071909. Pharmaceutics. 2023. PMID: 37514095 Free PMC article.
-
Efficacy and safety of lemborexant in subjects with insomnia disorder receiving medications for depression or anxiety symptoms.Neuropsychopharmacol Rep. 2025 Mar;45(1):e12509. doi: 10.1002/npr2.12509. Epub 2024 Dec 4. Neuropsychopharmacol Rep. 2025. PMID: 39632341 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

