Biological Reduction of a U(V)-Organic Ligand Complex
- PMID: 33705103
- PMCID: PMC8154365
- DOI: 10.1021/acs.est.0c06633
Biological Reduction of a U(V)-Organic Ligand Complex
Abstract
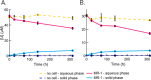
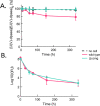
Metal-reducing microorganisms such as Shewanella oneidensis MR-1 reduce highly soluble species of hexavalent uranyl (U(VI)) to less mobile tetravalent uranium (U(IV)) compounds. The biologically mediated immobilization of U(VI) is being considered for the remediation of U contamination. However, the mechanistic underpinnings of biological U(VI) reduction remain unresolved. It has become clear that a first electron transfer occurs to form pentavalent (U(V)) intermediates, but it has not been definitively established whether a second one-electron transfer can occur or if disproportionation of U(V) is required. Here, we utilize the unusual properties of dpaea2- ((dpaeaH2═bis(pyridyl-6-methyl-2-carboxylate)-ethylamine)), a ligand forming a stable soluble aqueous complex with U(V), and investigate the reduction of U(VI)-dpaea and U(V)-dpaea by S. oneidensis MR-1. We establish U speciation through time by separating U(VI) from U(IV) by ion exchange chromatography and characterize the reaction end-products using U M4-edge high resolution X-ray absorption near-edge structure (HR-XANES) spectroscopy. We document the reduction of solid phase U(VI)-dpaea to aqueous U(V)-dpaea but, most importantly, demonstrate that of U(V)-dpaea to U(IV). This work establishes the potential for biological reduction of U(V) bound to a stabilizing ligand. Thus, further work is warranted to investigate the possible persistence of U(V)-organic complexes followed by their bioreduction in environmental systems.
Keywords: HERFD-XANES; Shewanella; bioremediation; disproportionation; electron transfer; pentavalent uranium; scanning transmission electron microscopy (STEM); uranium reduction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Lovley D. R.; Phillips E. J. P.; Gorby Y. A.; Landa E. R. Microbial Reduction of Uranium. Nature 1991, 350 (6317), 413–416. 10.1038/350413a0. - DOI
-
- Barton L. L.; Choudhury K.; Thomson B. M.; Steenhoudt K.; Groffman A. R. Bacterial Reduction of Soluble Uranium: The First Step of in Situ Immobilization of Uranium. Radioact. Waste Manag. Environ. Restor. 1996, 20 (2–3), 141–151.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

