High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology
- PMID: 33705116
- PMCID: PMC8154331
- DOI: 10.1021/acs.chemrev.0c00752
High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology
Abstract
The complex interaction of cells with biomaterials (i.e., materiobiology) plays an increasingly pivotal role in the development of novel implants, biomedical devices, and tissue engineering scaffolds to treat diseases, aid in the restoration of bodily functions, construct healthy tissues, or regenerate diseased ones. However, the conventional approaches are incapable of screening the huge amount of potential material parameter combinations to identify the optimal cell responses and involve a combination of serendipity and many series of trial-and-error experiments. For advanced tissue engineering and regenerative medicine, highly efficient and complex bioanalysis platforms are expected to explore the complex interaction of cells with biomaterials using combinatorial approaches that offer desired complex microenvironments during healing, development, and homeostasis. In this review, we first introduce materiobiology and its high-throughput screening (HTS). Then we present an in-depth of the recent progress of 2D/3D HTS platforms (i.e., gradient and microarray) in the principle, preparation, screening for materiobiology, and combination with other advanced technologies. The Compendium for Biomaterial Transcriptomics and high content imaging, computational simulations, and their translation toward commercial and clinical uses are highlighted. In the final section, current challenges and future perspectives are discussed. High-throughput experimentation within the field of materiobiology enables the elucidation of the relationships between biomaterial properties and biological behavior and thereby serves as a potential tool for accelerating the development of high-performance biomaterials.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.v.R is cofounder/scientific advisor/shareholder of BiomACS BV. There are no other conflicts to declare.
Figures










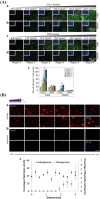

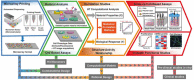








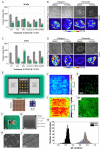


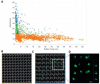









References
-
- Lanza R.; Langer R.; Vacanti J. P.. Principles of Tissue Engineering; Academic Press, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

