PARP inhibitors in breast and ovarian cancer with BRCA mutations: a meta-analysis of survival
- PMID: 33705352
- PMCID: PMC8034970
- DOI: 10.18632/aging.202724
PARP inhibitors in breast and ovarian cancer with BRCA mutations: a meta-analysis of survival
Abstract
Objective: To evaluate the efficacy of poly ADP ribose polymerase (PARP) inhibitors (PARPis) in breast and ovarian cancer with BRCA (BReast CAncer susceptibility gene) mutation (BRCAm).
Methods: We conducted a meta-analysis of randomized controlled, phase II or III trials by searching of electronic databases from inception to September 1, 2020. The efficacy of PARPis measured by hazard ratios (HRs) and 95% confidence intervals (95% CIs) for progression free survival (PFS) and overall survival (OS) of patients.
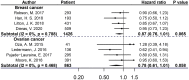
Results: By addition of PARPis to conventional therapy, breast or ovarian cancer patients carrying BRCAm significantly benefited PFS (breast cancer: HR 0.64, 95% CI=0.55-0.75, P<0.001; ovarian cancer: HR 0.33, 95% CI=0.27-0.42, P<0.001), but OS of patients did not increase significantly in these two cancer types (breast cancer: HR 0.87, 95% CI=0.76-1.01, P=0.065; ovarian cancer: HR 0.78, 95% CI=0.61-1.01, P=0.058). For ovarian cancer patients carrying BRCAm, the use of therapy with PARPis yielded longer PFS at the stage of newly diagnosed than the stage of recurrence (22.5 months vs 9.6 months).
Conclusion: PARPis were beneficial to all with BRCAm, but they were "most" beneficial to the ovarian cancer subset when administered early after diagnosis, rather than after recurrence.
Keywords: BRCA mutation; PARP inhibitor; breast cancer; efficacy; ovarian cancer.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

