Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva
- PMID: 33705358
- PMCID: PMC8119212
- DOI: 10.1172/jci.insight.95042
Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva
Abstract
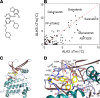
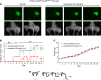
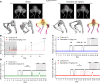
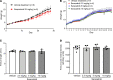
Currently, no effective therapies exist for fibrodysplasia ossificans progressiva (FOP), a rare congenital syndrome in which heterotopic bone is formed in soft tissues owing to dysregulated activity of the bone morphogenetic protein (BMP) receptor kinase ALK2 (also known as ACVR1). From a screen of known biologically active compounds, we identified saracatinib as a potent ALK2 kinase inhibitor. In enzymatic and cell-based assays, saracatinib preferentially inhibited ALK2, compared with other receptors of the BMP/TGF-β signaling pathway, and induced dorsalization in zebrafish embryos consistent with BMP antagonism. We further tested the efficacy of saracatinib using an inducible ACVR1Q207D-transgenic mouse line, which provides a model of heterotopic ossification (HO), as well as an inducible ACVR1R206H-knockin mouse, which serves as a genetically and physiologically faithful FOP model. In both models, saracatinib was well tolerated and potently inhibited the development of HO, even when administered transiently following soft tissue injury. Together, these data suggest that saracatinib is an efficacious clinical candidate for repositioning in FOP treatment, offering an accelerated path to clinical proof-of-efficacy studies and potentially significant benefits to individuals with this devastating condition.
Keywords: Bone Biology; Drug screens; Growth factors; Protein kinases; Therapeutics.
Conflict of interest statement
Figures


References
-
- Ekins S, et al. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov Today. 2011;16(7–8):298–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

