Expansion microscopy provides new insights into the cytoskeleton of malaria parasites including the conservation of a conoid
- PMID: 33705377
- PMCID: PMC7951857
- DOI: 10.1371/journal.pbio.3001020
Expansion microscopy provides new insights into the cytoskeleton of malaria parasites including the conservation of a conoid
Erratum in
-
Correction: Expansion microscopy provides new insights into the cytoskeleton of malaria parasites including the conservation of a conoid.PLoS Biol. 2021 Sep 14;19(9):e3001401. doi: 10.1371/journal.pbio.3001401. eCollection 2021 Sep. PLoS Biol. 2021. PMID: 34520452 Free PMC article.
Abstract
Malaria is caused by unicellular Plasmodium parasites. Plasmodium relies on diverse microtubule cytoskeletal structures for its reproduction, multiplication, and dissemination. Due to the small size of this parasite, its cytoskeleton has been primarily observable by electron microscopy (EM). Here, we demonstrate that the nanoscale cytoskeleton organisation is within reach using ultrastructure expansion microscopy (U-ExM). In developing microgametocytes, U-ExM allows monitoring the dynamic assembly of axonemes and concomitant tubulin polyglutamylation in whole cells. In the invasive merozoite and ookinete forms, U-ExM unveils the diversity across Plasmodium stages and species of the subpellicular microtubule arrays that confer cell rigidity. In ookinetes, we additionally identify an apical tubulin ring (ATR) that colocalises with markers of the conoid in related apicomplexan parasites. This tubulin-containing structure was presumed to be lost in Plasmodium despite its crucial role in motility and invasion in other apicomplexans. Here, U-ExM reveals that a divergent and considerably reduced form of the conoid is actually conserved in Plasmodium species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
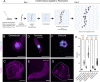
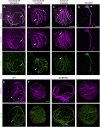
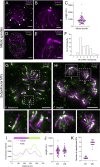
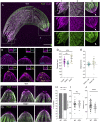

Comment in
-
Apicomplexans: A conoid ring unites them all.PLoS Biol. 2021 Mar 11;19(3):e3001105. doi: 10.1371/journal.pbio.3001105. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33705378 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

