Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: A cost-effectiveness analysis
- PMID: 33705382
- PMCID: PMC7951902
- DOI: 10.1371/journal.pmed.1003534
Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: A cost-effectiveness analysis
Abstract
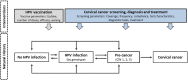
Background: A nonavalent human papillomavirus (HPV) vaccine has been licensed for use in women and men up to age 45 years in the United States. The cost-effectiveness of HPV vaccination for women and men aged 30 to 45 years in the context of cervical cancer screening practice was evaluated to inform national guidelines.
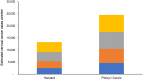
Methods and findings: We utilized 2 independent HPV microsimulation models to evaluate the cost-effectiveness of extending the upper age limit of HPV vaccination in women (from age 26 years) and men (from age 21 years) up to age 30, 35, 40, or 45 years. The models were empirically calibrated to reflect the burden of HPV and related cancers in the US population and used standardized inputs regarding historical and future vaccination uptake, vaccine efficacy, cervical cancer screening, and costs. Disease outcomes included cervical, anal, oropharyngeal, vulvar, vaginal, and penile cancers, as well as genital warts. Both models projected higher costs and greater health benefits as the upper age limit of HPV vaccination increased. Strategies of vaccinating females and males up to ages 30, 35, and 40 years were found to be less cost-effective than vaccinating up to age 45 years, which had an incremental cost-effectiveness ratio (ICER) greater than a commonly accepted upper threshold of $200,000 per quality-adjusted life year (QALY) gained. When including all HPV-related outcomes, the ICER for vaccinating up to age 45 years ranged from $315,700 to $440,600 per QALY gained. Assumptions regarding cervical screening compliance, vaccine costs, and the natural history of noncervical HPV-related cancers had major impacts on the cost-effectiveness of the vaccination strategies. Key limitations of the study were related to uncertainties in the data used to inform the models, including the timing of vaccine impact on noncervical cancers and vaccine efficacy at older ages.
Conclusions: Our results from 2 independent models suggest that HPV vaccination for adult women and men aged 30 to 45 years is unlikely to represent good value for money in the US.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: KC is the co-principal investigator of Compass (NCT02328872), an unrelated trial of cervical screening, which is conducted and funded by the VCS Foundation, a government-funded health promotion charity. The VCS Foundation has received equipment and a funding contribution for the Compass trial from Roche Molecular Systems but neither KC nor her institution on her behalf have received funding for this trial or any other project. MAS receives salary support from the National Health & Medical Research Council (Australia) and Cancer Institute NSW. KTS receives salary support from the Cancer Institute NSW. All other authors declare no conflicts.
Figures
References
-
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24. . - PubMed
-
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30. Epub 2014/08/29. . - PubMed
-
- Ault KA, Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861–8. 10.1016/S0140-6736(07)60852-6 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

