Bacterial Mucosal Immunotherapy with MV130 Prevents Recurrent Wheezing in Children: A Randomized, Double-Blind, Placebo-controlled Clinical Trial
- PMID: 33705665
- PMCID: PMC8480240
- DOI: 10.1164/rccm.202003-0520OC
Bacterial Mucosal Immunotherapy with MV130 Prevents Recurrent Wheezing in Children: A Randomized, Double-Blind, Placebo-controlled Clinical Trial
Abstract
Rationale: Recurrent wheezing in children represents a severe public health concern. Wheezing attacks (WA), mainly associated with viral infections, lack effective preventive therapies. Objectives: To evaluate the efficacy and safety of mucosal sublingual immunotherapy based on whole inactivated bacteria (MV130) in preventing WA in children. Methods: A Phase 3 randomized, double-blind, placebo-controlled, parallel-group trial including a cohort of 120 children <3 years old with ⩾3 WA during the previous year was conducted. Children with a positive skin test to common aeroallergens in the area where the clinical trial was performed were excluded from the trial. Subjects received MV130 or placebo daily for 6 months. The primary endpoint was the number of WA within 1 year after the first dose comparing MV130 and placebo. Measurements and Main Results: There was a significant lower number of WA in MV130 versus the placebo group, 3.0 (interquartile range [IQR], 2.0-4.0) versus 5.0 (IQR, 3.0-7.0) (P < 0.001). As secondary outcomes, a decrease in the duration of WA and a reduction in symptoms and medication scores in the MV130 versus placebo group were found. No adverse events were reported related to the active treatment. Conclusions: Mucosal bacterial immunotherapy with MV130 shows safety and clinical efficacy against recurrent WA in children.Clinical trial registered with www.clinicaltrials.gov (NCT01734811).
Keywords: MV130; clinical trial; mucosal vaccination; wheezing attacks.
Figures
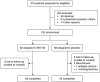
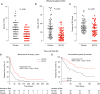
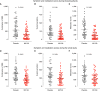
Comment in
-
Innate Immune Training for Prevention of Recurrent Wheeze in Early Childhood.Am J Respir Crit Care Med. 2021 Aug 15;204(4):392-394. doi: 10.1164/rccm.202103-0698ED. Am J Respir Crit Care Med. 2021. PMID: 33844949 Free PMC article. No abstract available.
References
-
- Camargo CA, Jr, Rachelefsky G, Schatz M. Managing asthma exacerbations in the emergency department: summary of the National Asthma Education and Prevention Program Expert Panel Report 3 guidelines for the management of asthma exacerbations. J Emerg Med . 2009;37(Suppl):S6–S17. - PubMed
-
- Meissner HC. Viral bronchiolitis in children. N Engl J Med . 2016;374:62–72. - PubMed
-
- Alvarez-Alvarez I, Niu H, Guillen-Grima F, Aguinaga-Ontoso I. Meta-analysis of prevalence of wheezing and recurrent wheezing in infants. Allergol Immunopathol (Madr) . 2018;46:210–217. - PubMed
-
- Garcia-Marcos L, Mallol J, Solé D, Brand PL. EISL Study Group. International study of wheezing in infants: risk factors in affluent and non-affluent countries during the first year of life. Pediatr Allergy Immunol . 2010;21:878–888. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

