Dissecting the contribution of host genetics and the microbiome in complex behaviors
- PMID: 33705688
- PMCID: PMC8996745
- DOI: 10.1016/j.cell.2021.02.009
Dissecting the contribution of host genetics and the microbiome in complex behaviors
Abstract
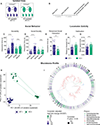
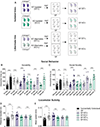
The core symptoms of many neurological disorders have traditionally been thought to be caused by genetic variants affecting brain development and function. However, the gut microbiome, another important source of variation, can also influence specific behaviors. Thus, it is critical to unravel the contributions of host genetic variation, the microbiome, and their interactions to complex behaviors. Unexpectedly, we discovered that different maladaptive behaviors are interdependently regulated by the microbiome and host genes in the Cntnap2-/- model for neurodevelopmental disorders. The hyperactivity phenotype of Cntnap2-/- mice is caused by host genetics, whereas the social-behavior phenotype is mediated by the gut microbiome. Interestingly, specific microbial intervention selectively rescued the social deficits in Cntnap2-/- mice through upregulation of metabolites in the tetrahydrobiopterin synthesis pathway. Our findings that behavioral abnormalities could have distinct origins (host genetic versus microbial) may change the way we think about neurological disorders and how to treat them.
Keywords: L. reuteri; gut-brain-axis; hologenome; hyperactivity; neurological disorders; oxytocin; social behavior; tetrahydrobiopterin.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A patent application related to the role of L. reuteri on social behavior has been filed by BCM. Findings regarding the manipulation of endogenous biotperin levels by the microbiome are the subject of a provisional patent application owned by BCM. M.C.-M. is a scientific co-founder of Mirkrovia. The authors declare no other competing interests.
Figures





Comment in
-
Bugs R Us: Restoring sociability with microbiota in autism.Cell Rep Med. 2021 Apr 20;2(4):100256. doi: 10.1016/j.xcrm.2021.100256. eCollection 2021 Apr 20. Cell Rep Med. 2021. PMID: 33948583 Free PMC article.
-
Host genetics, the microbiome & behaviour-a 'Holobiont' perspective.Cell Res. 2021 Aug;31(8):832-833. doi: 10.1038/s41422-021-00512-x. Cell Res. 2021. PMID: 33990786 Free PMC article. No abstract available.
References
-
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®) (American Psychiatric Pub; ).
-
- Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, et al. (2008). Molecular Cytogenetic Analysis and Resequencing of Contactin Associated Protein-Like 2 in Autism Spectrum Disorders. The American Journal of Human Genetics 82, 165–173. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

