Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis
- PMID: 33705690
- PMCID: PMC8049592
- DOI: 10.1016/S2214-109X(21)00026-7
Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis
Abstract
Background: A rapidly increasing number of serological surveys for antibodies to SARS-CoV-2 have been reported worldwide. We aimed to synthesise, combine, and assess this large corpus of data.
Methods: In this systematic review and meta-analysis, we searched PubMed, Embase, Web of Science, and five preprint servers for articles published in English between Dec 1, 2019, and Dec 22, 2020. Studies evaluating SARS-CoV-2 seroprevalence in humans after the first identified case in the area were included. Studies that only reported serological responses among patients with COVID-19, those using known infection status samples, or any animal experiments were all excluded. All data used for analysis were extracted from included papers. Study quality was assessed using a standardised scale. We estimated age-specific, sex-specific, and race-specific seroprevalence by WHO regions and subpopulations with different levels of exposures, and the ratio of serology-identified infections to virologically confirmed cases. This study is registered with PROSPERO, CRD42020198253.
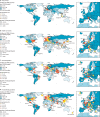
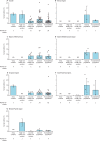
Findings: 16 506 studies were identified in the initial search, 2523 were assessed for eligibility after removal of duplicates and inappropriate titles and abstracts, and 404 serological studies (representing tests in 5 168 360 individuals) were included in the meta-analysis. In the 82 studies of higher quality, close contacts (18·0%, 95% CI 15·7-20·3) and high-risk health-care workers (17·1%, 9·9-24·4) had higher seroprevalence than did low-risk health-care workers (4·2%, 1·5-6·9) and the general population (8·0%, 6·8-9·2). The heterogeneity between included studies was high, with an overall I2 of 99·9% (p<0·0001). Seroprevalence varied greatly across WHO regions, with the lowest seroprevalence of general populations in the Western Pacific region (1·7%, 95% CI 0·0-5·0). The pooled infection-to-case ratio was similar between the region of the Americas (6·9, 95% CI 2·7-17·3) and the European region (8·4, 6·5-10·7), but higher in India (56·5, 28·5-112·0), the only country in the South-East Asia region with data.
Interpretation: Antibody-mediated herd immunity is far from being reached in most settings. Estimates of the ratio of serologically detected infections per virologically confirmed cases across WHO regions can help provide insights into the true proportion of the population infected from routine confirmation data.
Funding: National Science Fund for Distinguished Young Scholars, Key Emergency Project of Shanghai Science and Technology Committee, Program of Shanghai Academic/Technology Research Leader, National Science and Technology Major project of China, the US National Institutes of Health.
Translation: For the Chinese translation of the abstract see Supplementary Materials section.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Update of
-
Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis.medRxiv [Preprint]. 2020 Oct 29:2020.09.11.20192773. doi: 10.1101/2020.09.11.20192773. medRxiv. 2020. Update in: Lancet Glob Health. 2021 May;9(5):e598-e609. doi: 10.1016/S2214-109X(21)00026-7. PMID: 32935122 Free PMC article. Updated. Preprint.
Comment in
-
COVID-19 serosurveys for public health decision making.Lancet Glob Health. 2021 May;9(5):e559-e560. doi: 10.1016/S2214-109X(21)00057-7. Epub 2021 Mar 8. Lancet Glob Health. 2021. PMID: 33705691 Free PMC article. No abstract available.
-
Has COVID peaked? Maybe, but it's too soon to be sure.Nature. 2021 Mar;591(7851):512-513. doi: 10.1038/d41586-021-00705-9. Nature. 2021. PMID: 33737737 No abstract available.
References
-
- WHO Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
-
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020;382:692–694. - PubMed
-
- Sughayer MA, Mansour A, Al Nuirat A, Souan L, Ghanem M, Siag M. Covid-19 seroprevalence rate in healthy blood donors from a community under strict lockdown measures. medRxiv. 2020 doi: 10.1101/2020.06.06.20123919. published online June 7, 2020. (preprint). - DOI
Uncited References
-
- Appa A, Takahashi S, Rodriguez-Barraquer I, et al. Universal PCR and antibody testing demonstrate little to no transmission of SARS-CoV-2 in a rural community. medRxiv. 2020 doi: 10.1101/2020.08.15.20175786. published online Aug 17. (preprint). - DOI
-
- McLaughlin C, Doll MK, Morrison KT, et al. High community SARS-CoV-2 antibody seroprevalence in a ski resort community, Blaine County, Idaho, US. Preliminary results. medRxiv. 2020 doi: 10.1101/2020.07.19.20157198. published online July 21. (preprint). - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

