International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma
- PMID: 33705745
- PMCID: PMC8169638
- DOI: 10.1053/j.gastro.2021.01.233
International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma
Keywords: Biomarkers; Cirrhosis; Liver Cancer.
Figures
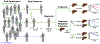


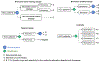
References
-
- Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217–1237.e3. - PubMed
-
- Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 1996;88:1456–1466. - PubMed
-
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005;97:1180–1184. - PubMed
-
- Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 2001;93:1054–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA226052/CA/NCI NIH HHS/United States
- R01 CA212008/CA/NCI NIH HHS/United States
- R01 CA230503/CA/NCI NIH HHS/United States
- U24 CA230144/CA/NCI NIH HHS/United States
- R01 CA222900/CA/NCI NIH HHS/United States
- U24 CA086368/CA/NCI NIH HHS/United States
- U01 CA230694/CA/NCI NIH HHS/United States
- 26813/CRUK_/Cancer Research UK/United Kingdom
- 671231/ERC_/European Research Council/International
- U01 CA230669/CA/NCI NIH HHS/United States
- C9380/A26813/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- R01 CA233794/CA/NCI NIH HHS/United States
- P30 CA196521/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

