Loss of sarcomeric proteins via upregulation of JAK/STAT signaling underlies interferon-γ-induced contractile deficit in engineered human myocardium
- PMID: 33705988
- PMCID: PMC8096718
- DOI: 10.1016/j.actbio.2021.03.007
Loss of sarcomeric proteins via upregulation of JAK/STAT signaling underlies interferon-γ-induced contractile deficit in engineered human myocardium
Abstract
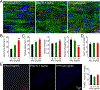
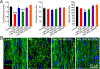
The level of circulating interferon-γ (IFNγ) is elevated in various clinical conditions including autoimmune and inflammatory diseases, sepsis, acute coronary syndrome, and viral infections. As these conditions are associated with high risk of myocardial dysfunction, we investigated the effects of IFNγ on 3D fibrin-based engineered human cardiac tissues ("cardiobundles"). Cardiobundles were fabricated from human pluripotent stem cell-derived cardiomyocytes, exposed to 0-20 ng/ml of IFNγ on culture days 7-14, and assessed for changes in tissue structure, viability, contractile force and calcium transient generation, action potential propagation, cytokine secretion, and expression of select genes and proteins. We found that application of IFNγ induced a dose-dependent reduction in contractile force generation, deterioration of sarcomeric organization, and cardiomyocyte disarray, without significantly altering cell viability, action potential propagation, or calcium transient amplitude. At molecular level, the IFNγ-induced structural and functional deficits could be attributed to altered balance of pro- and anti-inflammatory cytokines, upregulation of JAK/STAT signaling pathway (JAK1, JAK2, and STAT1), and reduced expression of myosin heavy chain, myosin light chain-2v, and sarcomeric α-actinin. Application of clinically used JAK/STAT inhibitors, tofacitinib and baricitinib, fully prevented IFNγ-induced cardiomyopathy, confirming the critical roles of this signaling pathway in inflammatory cardiac disease. Taken together, our in vitro studies in engineered myocardial tissues reveal direct adverse effects of pro-inflammatory cytokine IFNγ on human cardiomyocytes and establish the foundation for a potential use of cardiobundle platform in modeling of inflammatory myocardial disease and therapy. STATEMENT OF SIGNIFICANCE: Various inflammatory and autoimmune diseases including rheumatoid arthritis, sepsis, lupus erythematosus, Chagas disease, and others, as well as viral infections including H1N1 influenza and COVID-19 show increased systemic levels of a pro-inflammatory cytokine interferon-γ (IFNγ) and are associated with high risk of heart disease. Here we explored for the first time if chronically elevated levels of IFNγ can negatively affect structure and function of engineered human heart tissues in vitro. Our studies revealed IFNγ-induced deterioration of myofibrillar organization and contractile force production in human cardiomyocytes, attributed to decreased expression of multiple sarcomeric proteins and upregulation of JAK/STAT signaling pathway. FDA-approved JAK inhibitors fully blocked the adverse effects of IFNγ, suggesting a potentially effective strategy against human inflammatory cardiomyopathy.
Keywords: COVID-19; Fibrin hydrogel; Inflammation; Secretome; hiPSC.
Copyright © 2021 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


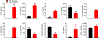


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

