ASSURED-SQVM diagnostics for COVID-19: addressing the why, when, where, who, what and how of testing
- PMID: 33706663
- PMCID: PMC8006264
- DOI: 10.1080/14737159.2021.1902311
ASSURED-SQVM diagnostics for COVID-19: addressing the why, when, where, who, what and how of testing
Abstract
Introduction: SARS-CoV-2, the new coronavirus that originated in 2019, continues to impact every aspect of society in a profound manner. Testing will remain an important tool to mitigate the effects of this pandemic as early and accurate diagnosis can lead to appropriate countermeasures to reduce mortality and morbidity. However, testing isn't a simple yes/no answer as the target and host are complex, the virus is a moving target, there is a plethora of tests that identify different parts of the virus and have their own limits and range of detection, and when prevalence is low, false positives and negatives can be very high.Areas covered: This article covers all the major questions related to COVID-19 diagnostics, the why, when, where, who, what and how of testing, the different types of tests, interpretation of results and the ideal ASSURED-SQVM diagnostic. A comprehensive literature review using all the publicly available databases and government websites and reports was performed.Expert opinion: Diagnostics that meet the 'ASSURED-SQVM' (Affordable, Selective and Sensitive, User-friendly, Rapid and Robust, Equipment-free, Deliverable to end-users and additionally, allows for Self-testing, Quantifiable, detects if pathogens are Viable and can detect Multiple pathogens) would make a major impact in our fight against the current pandemic. While a significant majority of researchers focus on developing novel diagnostics that are highly selective and sensitive, it is the opinion of these authors that other aspects of the ASSURED-SQVM principles also be considered early in the development process for widespread use.
Keywords: ASSURED-SQVM; Covid-19; PCR; SARS-CoV-2; diagnostics; influenza; pandemic; rapid diagnostics.
Figures
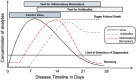



References
-
- World Health Organization . Coronavirus Disease. 2020; Available from: https://covid19.who.int/.
-
- Chen N, Zhou M, Dong X, et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395(10223): 507–513. 2020. . - PMC - PubMed
-
•• This article was one of the first articles that described the epidemiological, demographic, clinical, and radiological features and laboratory data of patients infected with the novel coronavirus.
-
- Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from coronavirus disease 2019 (COVID-19) infection. Ann Acad Med Singapore. 2020;49(3):108–118. . - PubMed
-
- Thanh Le T, Andreadakis Z, Kumar A, et al., The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 19(5): 305–306. 2020. . - PubMed
-
•• This article covers the major vaccine efforts to combat the virus.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
