Fetal inflammation induces acute immune tolerance in the neonatal rat hippocampus
- PMID: 33706765
- PMCID: PMC7953777
- DOI: 10.1186/s12974-021-02119-w
Fetal inflammation induces acute immune tolerance in the neonatal rat hippocampus
Abstract
Background: Infants born preterm due to chorioamnionitis are frequently affected by a fetal inflammatory response syndrome (FIRS) and then by subsequent postnatal infections. FIRS and postnatal systemic inflammatory events independently contribute to poor neurocognitive outcomes of preterm infants. Developmental integrity of the hippocampus is crucial for intact neurocognitive outcomes in preterms and hippocampally dependent behaviors are particularly vulnerable to preterm systemic inflammation. How FIRS modulates the hippocampal immune response to acute postnatal inflammatory events is not well understood.
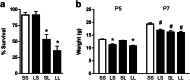
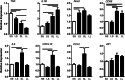
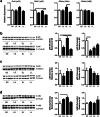
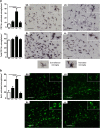
Methods: Prenatal LPS exposed (FIRS) and control neonatal rats received i.p. LPS or saline at postnatal day (P) 5. On P7, immune response was evaluated in the hippocampus of four treatment groups by measuring gene expression of inflammatory mediators and cytosolic and nuclear NFκB pathway proteins. Microglial activation was determined by CD11b+ and Iba1+ immunohistochemistry (IHC) and inflammatory gene expression of isolated microglia. Astrocyte reactivity was measured using Gfap+ IHC.
Results: Postnatal LPS resulted in a robust hippocampal inflammatory response. In contrast, FIRS induced by prenatal LPS attenuated the response to postnatal LPS exposure, evidenced by decreased gene expression of inflammatory mediators, decreased nuclear NFκB p65 protein, and fewer activated CD11b+ and Iba1+ microglia. Isolated microglia demonstrated inflammatory gene upregulation to postnatal LPS without evidence of immune tolerance by prenatal LPS.
Conclusion: Prenatal LPS exposure induced immune tolerance to subsequent postnatal LPS exposure in the hippocampus. Microglia demonstrate a robust inflammatory response to postnatal LPS, but only a partial immune tolerance response.
Keywords: Fetal inflammation; Hippocampus; Immune tolerance; LPS; Microglial activation; Preterm.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: Final Data for 2014. Natl Vital Stat Rep. 2015;64:1–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

