Towards a sensitive and accurate interpretation of molecular testing for SARS-CoV-2: a rapid review of 264 studies
- PMID: 33706863
- PMCID: PMC7953531
- DOI: 10.2807/1560-7917.ES.2021.26.10.2001134
Towards a sensitive and accurate interpretation of molecular testing for SARS-CoV-2: a rapid review of 264 studies
Abstract
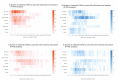
BackgroundSensitive molecular diagnostics and correct test interpretation are crucial for accurate COVID-19 diagnosis and thereby essential for good clinical practice. Furthermore, they are a key factor in outbreak control where active case finding in combination with isolation and contact tracing are crucial.AimWith the objective to inform the public health and laboratory responses to the pandemic, we reviewed current published knowledge on the kinetics of SARS-CoV-2 infection as assessed by RNA molecular detection in a wide range of clinical samples.MethodsWe performed an extensive search on studies published between 1 December 2019 and 15 May 2020, reporting on molecular detection and/or isolation of SARS-CoV-2 in any human laboratory specimen.ResultsWe compiled a dataset of 264 studies including 32,515 COVID-19 cases, and additionally aggregated data points (n = 2,777) from sampling of 217 adults with known infection timeline. We summarised data on SARS-CoV-2 detection in the respiratory and gastrointestinal tract, blood, oral fluid, tears, cerebrospinal fluid, peritoneal fluid, semen, vaginal fluid; where provided, we also summarised specific observations on SARS-CoV-2 detection in pregnancy, infancy, children, adolescents and immunocompromised individuals.ConclusionOptimal SARS-CoV-2 molecular testing relies on choosing the most appropriate sample type, collected with adequate sampling technique, and with the infection timeline in mind. We outlined knowledge gaps and directions for future well-documented systematic studies.
Keywords: COVID-19; Laboratory Diagnosis; Polymerase Chain Reaction; SARS-CoV-2.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). Geneva: WHO; 2020. Updated 30 Jan 2020. [Accessed 07 May 2020]. Available from: https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-...
-
- World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Geneva: WHO; 2020. [Accessed 9 May 2020]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
