Dorsal commissural axon guidance in the developing spinal cord
- PMID: 33706918
- PMCID: PMC9109821
- DOI: 10.1016/bs.ctdb.2020.10.009
Dorsal commissural axon guidance in the developing spinal cord
Abstract
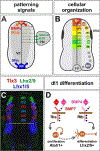
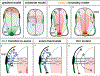
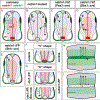
Commissural axons have been a key model system for identifying axon guidance signals in vertebrates. This review summarizes the current thinking about the molecular and cellular mechanisms that establish a specific commissural neural circuit: the dI1 neurons in the developing spinal cord. We assess the contribution of long- and short-range signaling while sequentially following the developmental timeline from the birth of dI1 neurons, to the extension of commissural axons first circumferentially and then contralaterally into the ventral funiculus.
Keywords: Axon guidance; BMP; Chemotaxis; Commissural axons; Ephrin; Floor plate; Haptotaxis; Netrin1; Neural development; Robo; Roof plate; Slit.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Long-Range Guidance of Spinal Commissural Axons by Netrin1 and Sonic Hedgehog from Midline Floor Plate Cells.Neuron. 2019 Feb 20;101(4):635-647.e4. doi: 10.1016/j.neuron.2018.12.025. Epub 2019 Jan 17. Neuron. 2019. PMID: 30661738
-
Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord.Neuron. 2017 May 17;94(4):790-799.e3. doi: 10.1016/j.neuron.2017.03.007. Epub 2017 Apr 21. Neuron. 2017. PMID: 28434801 Free PMC article.
-
Netrin1-DCC-Mediated Attraction Guides Post-Crossing Commissural Axons in the Hindbrain.J Neurosci. 2015 Aug 19;35(33):11707-18. doi: 10.1523/JNEUROSCI.0613-15.2015. J Neurosci. 2015. PMID: 26290247 Free PMC article.
-
The spinal cord shows the way - How axons navigate intermediate targets.Dev Biol. 2017 Dec 1;432(1):43-52. doi: 10.1016/j.ydbio.2016.12.002. Epub 2016 Dec 10. Dev Biol. 2017. PMID: 27965053 Review.
-
Commissural axon guidance in the developing spinal cord: from Cajal to the present day.Neural Dev. 2019 Sep 12;14(1):9. doi: 10.1186/s13064-019-0133-1. Neural Dev. 2019. PMID: 31514748 Free PMC article. Review.
Cited by
-
Guidance landscapes unveiled by quantitative proteomics to control reinnervation in adult visual system.Nat Commun. 2022 Oct 13;13(1):6040. doi: 10.1038/s41467-022-33799-4. Nat Commun. 2022. PMID: 36229455 Free PMC article.
-
MYC regulation of the miR-92-Robo1 axis in Slit-mediated commissural axon guidance.Mol Biol Cell. 2025 Apr 1;36(4):ar50. doi: 10.1091/mbc.E24-12-0534. Epub 2025 Feb 28. Mol Biol Cell. 2025. PMID: 40020181 Free PMC article.
-
Integrin-mediated electric axon guidance underlying optic nerve formation in the embryonic chick retina.Commun Biol. 2023 Jun 30;6(1):680. doi: 10.1038/s42003-023-05056-x. Commun Biol. 2023. PMID: 37391492 Free PMC article.
-
Netrin1 patterns the dorsal spinal cord through modulation of Bmp signaling.Cell Rep. 2024 Nov 26;43(11):114954. doi: 10.1016/j.celrep.2024.114954. Epub 2024 Nov 14. Cell Rep. 2024. PMID: 39547237 Free PMC article.
References
-
- Alther TA, Domanitskaya E, & Stoeckli ET (2016). Calsyntenin 1-mediated trafficking of axon guidance receptors regulates the switch in axonal responsiveness at a choice point. Development (Cambridge, England), 143, 994–1004. - PubMed
-
- Altman J, & Bayer SA (1984). The development of the rat spinal cord. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

