Ontogeny of adult neural stem cells in the mammalian brain
- PMID: 33706926
- PMCID: PMC8363052
- DOI: 10.1016/bs.ctdb.2020.11.002
Ontogeny of adult neural stem cells in the mammalian brain
Abstract
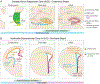
Neural stem cells (NSCs) persist into adulthood in the subgranular zone (SGZ) of the dentate gyrus in the hippocampus and in the ventricular-subventricular zone (V-SVZ) of the lateral ventricles, where they generate new neurons and glia cells that contribute to neural plasticity. A better understanding of the developmental process that enables NSCs to persist beyond development will provide insight into factors that determine the size and properties of the adult NSC pool and thus the capacity for life-long neurogenesis in the adult mammalian brain. We review current knowledge regarding the developmental origins of adult NSCs and the developmental process by which embryonic NSCs transition into their adult form. We also discuss potential mechanisms that might regulate proper establishment of the adult NSC pool, and propose future directions of research that will be key to unraveling how NSCs transform to establish the adult NSC pool in the mammalian brain.
Keywords: Dentate gyrus; Neural stem cells; Neurogenesis; Niche; Origin; Quiescence; Subgranular zone; Subventricular zone.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures


References
-
- Altman J, & Bayer SA (1990). Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. The Journal of Comparative Neurology, 301, 325–342. - PubMed
-
- Alvarez-Buylla A, Kohwi M, Nguyen TM, & Merkle FT (2008). The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harbor Symposia on Quantitative Biology, 73, 357–365. - PubMed
-
- Andreu Z, Khan MA, González-Gómez P, Negueruela S, Hortigüela R, San Emeterio J, et al. (2015). The cyclin-dependent kinase inhibitor p27 kip1 regulates radial stem cell quiescence and neurogenesis in the adult hippocampus. Stem Cells, 33, 219–229. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

