Caspase Signaling in ED Patients and Animal Models
- PMID: 33707045
- PMCID: PMC8068676
- DOI: 10.1016/j.jsxm.2021.01.175
Caspase Signaling in ED Patients and Animal Models
Abstract
Background: Current treatments for erectile dysfunction (ED) are ineffective in prostatectomy and diabetic patients due to cavernous nerve (CN) injury, which causes smooth muscle apoptosis, penile remodeling, and ED. Apoptosis can occur via the intrinsic (caspase 9) or extrinsic (caspase 8) pathway.
Aim: We examined the mechanism of how apoptosis occurs in ED patients and CN injury rat models to determine points of intervention for therapy development.
Methods and outcomes: Immunohistochemical and western analyses for caspase 3-cleaved, caspase-8 and caspase-9 (pro and active forms) were performed in corpora cavernosal tissue from Peyronie's, prostatectomy and diabetic ED patients (n = 33), penis from adult Sprague Dawley rats that underwent CN crush (n = 24), BB/WOR diabetic and control rats (n = 8), and aged rats (n = 9).
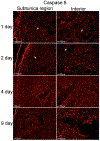
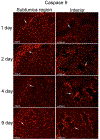
Results: Caspase 3-cleaved was observed in corpora cavernosa from Peyronie's patients and at higher abundance in prostatectomy and diabetic tissues. Apoptosis takes place primarily through the extrinsic (caspase 8) pathway in penis tissue of ED patients. In the CN crushed rat, caspase 3-cleaved was abundant from 1-9 days after injury, and apoptosis takes place primarily via the intrinsic (caspase 9) pathway. Caspase 9 was first observed and most abundant in a layer under the tunica, and after several days was observed in the lining of and between the sinuses of the corpora cavernosa. Caspase 8 was initially observed at low abundance in the rat corpora cavernosa and was not observed at later time points after CN injury. Aged and diabetic rat penis primarily exhibited intrinsic mechanisms, with diabetic rats also exhibiting mild extrinsic activation.
Clinical translation: Knowing how and when to intervene to prevent the apoptotic response most effectively is critical for the development of drugs to prevent ED, morphological remodeling of the corpora cavernosa, and thus, disease management.
Strengths and limitations: Animal models may diverge from the signaling mechanisms observed in ED patients. While the rat utilizes primarily caspase 9, there is a significant flux through caspase 8 early on, making it a reasonable model, as long as the timing of apoptosis is considered after CN injury.
Conclusions: Apoptosis takes place primarily through the extrinsic caspase 8 dependent pathway in ED patients and via the intrinsic caspase 9 dependent pathway in commonly used CN crush ED models. This is an important consideration for study design and interpretation that must be taken into account for therapy development and testing of drugs, and our therapeutic targets should ideally inhibit both apoptotic mechanisms. Martin S, Harrington DA, Ohlander S, et al. Caspase Signaling in ED Patients and Animal Models. J Sex Med 2021;18:711-722.
Keywords: Apoptosis; Caspase; Cavernous Nerve Injury; Erectile Dysfunction; Extrinsic; Intrinsic; Penis; Peripheral Nerve Regeneration.
Published by Elsevier Inc.
Figures





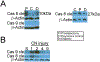


References
-
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994; 151: 54–61. - PubMed
-
- Heruti R, Shochat T, Tekes-Manova D, Ashkenazi I, Justo D (2004) Prevalence of erectile dysfunction among young adults: results of a large-scale 525 survey. J Sex Med 2004; 1: 284–291. - PubMed
-
- Nguyen HMT, Gabrielson AT, Hellstrom WJG. Erectile dysfunction in young men-A review of the prevalence and risk factors. Sex Med Rev 2017; 5: 508–520. - PubMed
-
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. American J Medicine 2007; 120: 151–157. - PubMed
-
- Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am 1996; 25:379–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

