A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine
- PMID: 33707061
- PMCID: PMC7871769
- DOI: 10.1016/j.vaccine.2021.02.007
A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine
Abstract
Background: Vaccines are urgently needed to prevent the global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We assessed the safety and immunogenicity of vaccine candidate mRNA-1273, encoding the prefusion-stabilized spike protein of SARS-CoV-2.
Methods: This phase 2, randomized, observer-blind, placebo-controlled trial was conducted at 8 sites in the USA, in healthy adults aged ≥18 years with no known history or risk of SARS-CoV-2 infection, and had not previously received an investigational CoV vaccine or treatment. Participants were stratified into two age cohorts (≥18-<55 and ≥55) and were randomly assigned (1:1:1) to either 50 or 100 µg of mRNA-1273, or placebo administered as two intramuscular injections 28 days apart. The primary outcomes were safety, reactogenicity, and immunogenicity assessed by anti-SARS-CoV-2-spike binding antibody level (bAb). Secondary outcome was immunogenicity assessed by SARS-CoV-2 neutralizing antibody (nAb) response.
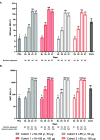
Results: Between 29 May and 8 July 2020, 600 participants were randomized, 300 per age cohort. The most common solicited adverse reactions were pain at injection site, headache, and fatigue following each vaccination in both age cohorts. One serious adverse event deemed unrelated by the site investigator occurred 33 days post-vaccination one. mRNA-1273 induced bAb and nAb by 28 days post-vaccination one that were higher at the 100 µg dose relative to the 50 µg dose; this difference was less apparent post-vaccination two. Binding antibodies and nAb increased substantially by 14 days following the second vaccination (day 43) to levels exceeding those of convalescent sera and remained elevated through day 57.
Conclusions: Vaccination with mRNA-1273 resulted in significant immune responses to SARS-CoV-2 in participants 18 years and older, with an acceptable safety profile, confirming the safety and immunogenicity of 50 and 100 µg mRNA-1273 given as a 2 dose-regimen. ClinicalTrials.gov; NCT04405076.
Keywords: COVID-19; Immunogenicity; Phase 2; SARS-CoV-2; Safety; Vaccine; mRNA-1273.
Copyright © 2021 Moderna Therapeutics. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Roderick McPhee, Wenmei Huang, Hamilton Bennett, Rolando Pajon, and Brett Leav are employees of Moderna, Inc., and may hold stock/stock options in the company. Biliana Nestorova is a contract employee of Moderna, Inc. Laurence Chu has no conflict of interest.
Figures


References
-
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. November 9, 2020. https://covid19.who.int/?gclid=CjwKCAjwps75BRAcEiwAEiACMVdInLBoj_EeG7jJA....
-
- Thanh Le T., Andreadakis Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M., et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:305–306. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

