Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis
- PMID: 33707445
- PMCID: PMC7952908
- DOI: 10.1038/s41467-021-21748-6
Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis
Abstract
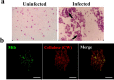
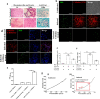
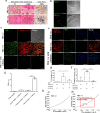
Tuberculosis is a chronic disease that displays several features commonly associated with biofilm-associated infections: immune system evasion, antibiotic treatment failures, and recurrence of infection. However, although Mycobacterium tuberculosis (Mtb) can form cellulose-containing biofilms in vitro, it remains unclear whether biofilms are formed during infection in vivo. Here, we demonstrate the formation of Mtb biofilms in animal models of infection and in patients, and that biofilm formation can contribute to drug tolerance. First, we show that cellulose is also a structural component of the extracellular matrix of in vitro biofilms of fast and slow-growing nontuberculous mycobacteria. Then, we use cellulose as a biomarker to detect Mtb biofilms in the lungs of experimentally infected mice and non-human primates, as well as in lung tissue sections obtained from patients with tuberculosis. Mtb strains defective in biofilm formation are attenuated for survival in mice, suggesting that biofilms protect bacilli from the host immune system. Furthermore, the administration of nebulized cellulase enhances the antimycobacterial activity of isoniazid and rifampicin in infected mice, supporting a role for biofilms in phenotypic drug tolerance. Our findings thus indicate that Mtb biofilms are relevant to human tuberculosis.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

