Synthesis and characterization of Cu(OH)2-NWs-PVA-AC Nano-composite and its use as an efficient adsorbent for removal of methylene blue
- PMID: 33707529
- PMCID: PMC7970965
- DOI: 10.1038/s41598-021-84797-3
Synthesis and characterization of Cu(OH)2-NWs-PVA-AC Nano-composite and its use as an efficient adsorbent for removal of methylene blue
Erratum in
-
Author Correction: Synthesis and characterization of Cu(OH)2-NWs-PVA-AC Nano-composite and its use as an efficient adsorbent for removal of methylene blue.Sci Rep. 2021 Aug 2;11(1):16031. doi: 10.1038/s41598-021-95555-w. Sci Rep. 2021. PMID: 34341482 Free PMC article. No abstract available.
Abstract
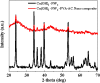
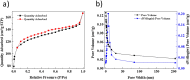
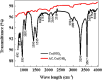
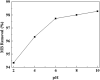
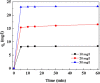
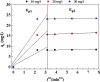
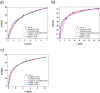
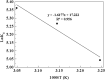
The present study focused on the synthesis of copper hydroxide nanowires decorated on activated carbon (Cu(OH)2-NWs-PVA-AC). The obtained Cu(OH)2-NWs-PVA-AC Nano-composite was distinguished by XRD, SEM, EDX, BET, FTIR and XPS respectively. Besides, different variables such as solution pH, and initial dye concentration, contact time, and temperature were performed on the adsorption efficiency of MB in a small batch reactor. Further, the experimental results are analyzed by various kinetic models via PFO, PSO, intra-particle diffusion and Elovich models, and the results revealed that among the kinetic models, PSO shows more suitability. In addition, different adsorption isotherms were applied to the obtained experimental data and found that Langmuir-Freundlich and Langmuir isotherm were best fits with the maximum adsorption capacity of 139.9 and 107.6 mg/g, respectively. The Nano-composite has outstanding MB removal efficiency of 94-98.5% with a span of 10 min. and decent adsorption of about 98.5% at a pH of 10. Thermodynamic constants like Gibbs free energy, entropy, and enthalpy were analyzed from the temperature reliance. The results reveal the adsorption processes are spontaneous and exothermic in nature. The high negative value of ΔG° (- 44.11 to - 48.86 kJ/mol) and a low negative value of ΔH° (- 28.96 kJ/mol) show the feasibility and exothermic nature of the adsorption process. The synthesized dye was found to be an efficient adsorbent for the potential removal of cationic dye (methylene blue) from wastewater within a short time.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bhatt AS, et al. Adsorption of an anionic dye from aqueous medium by organoclays: Equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012;2:8663–8671. doi: 10.1039/c2ra20347b. - DOI
-
- Alam MK, et al. Ultra-sensitive 2-nitrophenol detection based on reduced graphene oxide/ZnO nanocomposites. J. Electroanal. Chem. 2017;788:66–73. doi: 10.1016/j.jelechem.2017.02.004. - DOI
-
- Awual MR, et al. Facile mercury detection and removal from aqueous media involving ligand impregnated conjugate nanomaterials. Chem. Eng. J. 2016;290:243–251. doi: 10.1016/j.cej.2016.01.038. - DOI
-
- Lakkaboyana SK, Khantong S, Asmel NK, Yuzir A, Wan-Yaacob WZ. Synthesis of copper oxide nanowires-activated carbon (AC@CuO-NWs) and applied for removal methylene blue from aqueous solution: Kinetics, isotherms, and thermodynamics. J. Inorg. Organometall. Polym. Mater. 2019;29:1658–1668. doi: 10.1007/s10904-019-01128-w. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

