Function of γδ T cells in tumor immunology and their application to cancer therapy
- PMID: 33707742
- PMCID: PMC8080836
- DOI: 10.1038/s12276-021-00576-0
Function of γδ T cells in tumor immunology and their application to cancer therapy
Abstract
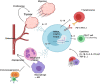
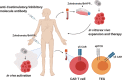
T cells of the γδ lineage are unconventional T cells with functions not restricted to MHC-mediated antigen presentation. Because of their broad antigen specificity and NK-like cytotoxicity, γδ T-cell importance in tumor immunology has been emphasized. However, some γδ T-cell subsets, especially those expressing IL-17, are immunosuppressive or tumor-promoting cells. Their cytokine profile and cytotoxicity are seemingly determined by cross-talk with microenvironment components, not by the γδTCR chain. Furthermore, much about the TCR antigen of γδ T cells remains unknown compared with the extreme diversity of their TCR chain pairs. Thus, the investigation and application of γδ T cells have been relatively difficult. Nevertheless, γδ T cells remain attractive targets for antitumor therapy because of their independence from MHC molecules. Because tumor cells have the ability to evade the immune system through MHC shedding, heterogeneous antigens, and low antigen spreading, MHC-independent γδ T cells represent good alternative targets for immunotherapy. Therefore, many approaches to using γδ T cells for antitumor therapy have been attempted, including induction of endogenous γδ T cell activation, adoptive transfer of expanded cells ex vivo, and utilization of chimeric antigen receptor (CAR)-T cells. Here, we discuss the function of γδ T cells in tumor immunology and their application to cancer therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Chien Y, et al. A third type of murine T-cell receptor gene. Nature. 1984;312:31–35. - PubMed
-
- Hayday AC, et al. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985;40:259–269. - PubMed
-
- Hayday ACGammadelta. T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. - PubMed
-
- Willcox, C. R., Mohammed, F. & Willcox, B. E. The distinct MHC-unrestricted immunobiology of innate-like and adaptive-like human gammadelta T cell subsets-Nature’s CAR-T cells. Immunol. Rev.298, 25–46 (2020). - PubMed
-
- Prinz I, Silva-Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur. J. Immunol. 2013;43:1988–1994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

