NLRP3 inflammasome in cancer and metabolic diseases
- PMID: 33707781
- PMCID: PMC8132572
- DOI: 10.1038/s41590-021-00886-5
NLRP3 inflammasome in cancer and metabolic diseases
Abstract
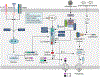
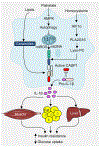
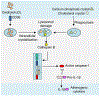
The NLRP3 inflammasome is a multimeric cytosolic protein complex that assembles in response to cellular perturbations. This assembly leads to the activation of caspase-1, which promotes maturation and release of the inflammatory cytokines interleukin-1β (IL-1β) and IL-18, as well as inflammatory cell death (pyroptosis). The inflammatory cytokines contribute to the development of systemic low-grade inflammation, and aberrant NLRP3 activation can drive a chronic inflammatory state in the body to modulate the pathogenesis of inflammation-associated diseases. Therefore, targeting NLRP3 or other signaling molecules downstream, such as caspase-1, IL-1β or IL-18, has the potential for great therapeutic benefit. However, NLRP3 inflammasome-mediated inflammatory cytokines play dual roles in mediating human disease. While they are detrimental in the pathogenesis of inflammatory and metabolic diseases, they have a beneficial role in numerous infectious diseases and some cancers. Therefore, fine tuning of NLRP3 inflammasome activity is essential for maintaining proper cellular homeostasis and health. In this Review, we will cover the mechanisms of NLRP3 inflammasome activation and its divergent roles in the pathogenesis of inflammation-associated diseases such as cancer, atherosclerosis, diabetes and obesity, highlighting the therapeutic potential of targeting this pathway.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures
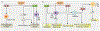
References
-
- Medzhitov R Origin and physiological roles of inflammation. Nature 454, 428–435 (2008). - PubMed
-
- Takeuchi O & Akira S Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010). - PubMed
-
- Kanneganti TD et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R35 CA253095/CA/NCI NIH HHS/United States
- AR056296/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI101935/AI/NIAID NIH HHS/United States
- AI124346/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 AI124346/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

